Trifluridine compound and medicine composition thereof
A compound and composition technology, applied in the field of new Trifluridine crystal form and its preparation method and pharmaceutical composition, can solve the problems of no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
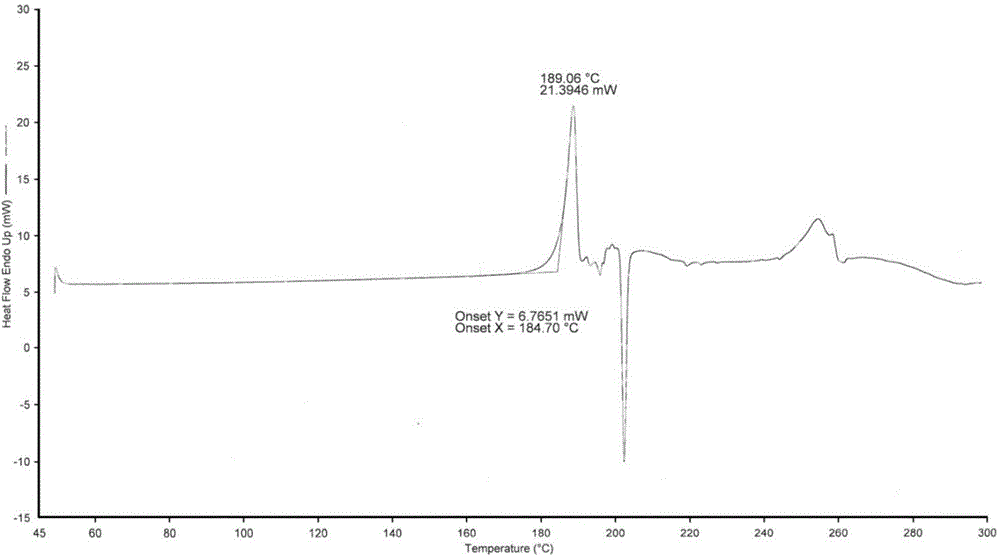
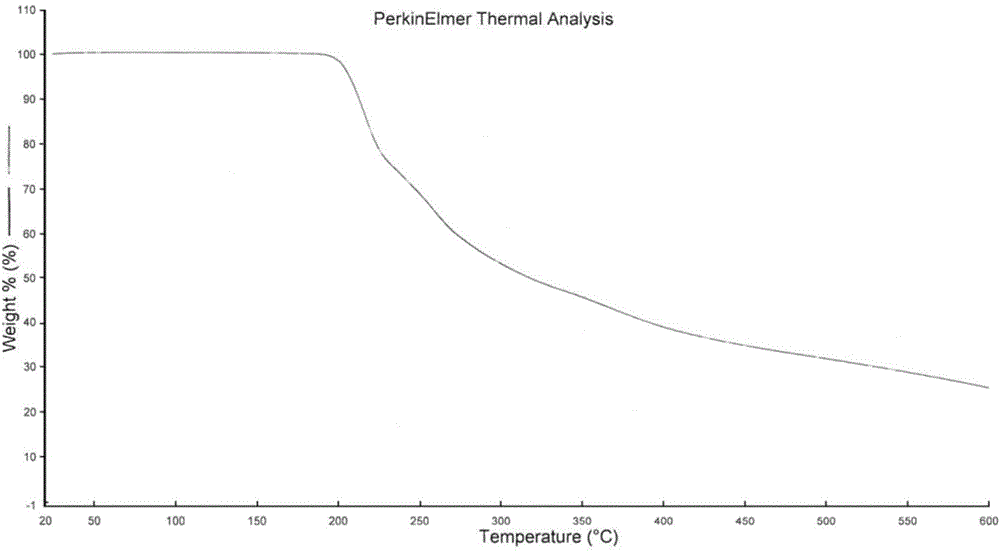
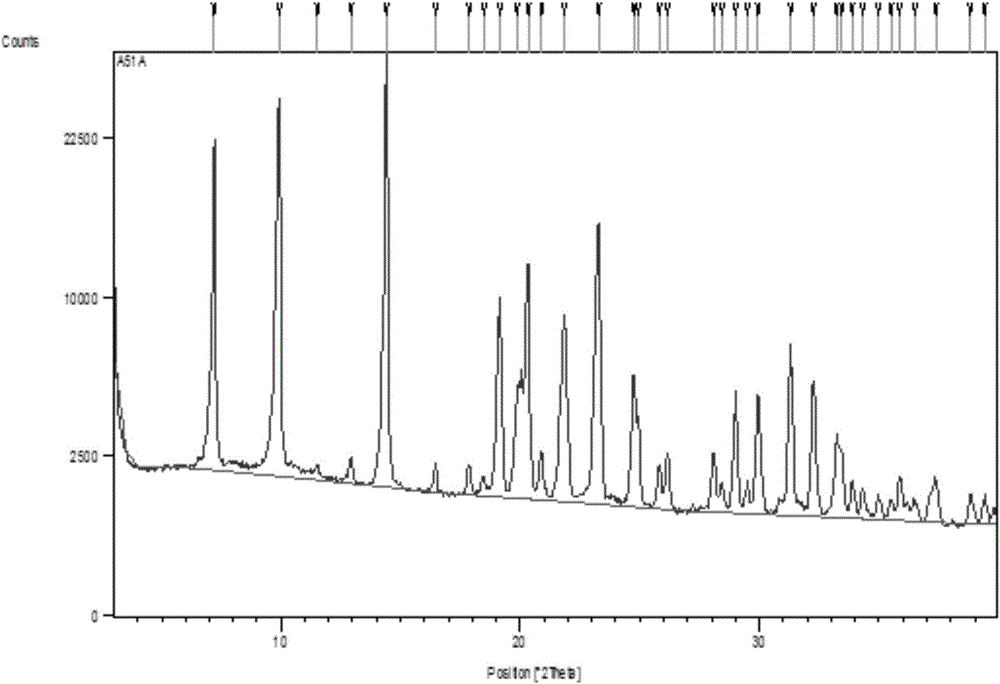
[0034]Add 1.5g (5.07mmol) Trifluridine primary product into 10mL methyl acetate, beat at 20°C for 20min, filter, add the filtered solid into 3mL mixed solvent of water and methanol (1:1 V / V), heat to 60°C The solid was fully dissolved until clear, then cooled to 10° C., left to stand for crystallization for 3 hours, filtered, collected and dried to obtain 1.06 g of white solid, yield: 70.7%.
Embodiment 2
[0036] Add 1.5g (5.07mmol) Trifluridine primary product into a mixed solvent composed of 7.5mL ethyl acetate and 7.5mL n-heptane, beat at 25°C for 30min, filter, add the filtered solid to water and ethanol (2:1V / V ) in 3mL mixed solvent, heated to 65°C to fully dissolve the solid until clear, then cooled to 16°C, stood for crystallization for 3.5h, filtered, collected the solid, and dried to obtain 1.13g of white solid, yield: 75.3%.
Embodiment 3
[0038] Add 1.5g (5.07mmol) Trifluridine primary product into a mixed solvent composed of 5.7mL butyl acetate and 14.3mL n-hexane, beat at 30°C for 45min, filter, add the filtered solid to water and n-butanol (3:1V / V ) in 3mL mixed solvent, heated to 75°C to fully dissolve the solid until clear, then cooled to 20°C, stood for crystallization for 3.5h, filtered, collected the solid, and dried to obtain 1.1g of white solid, yield: 73.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com