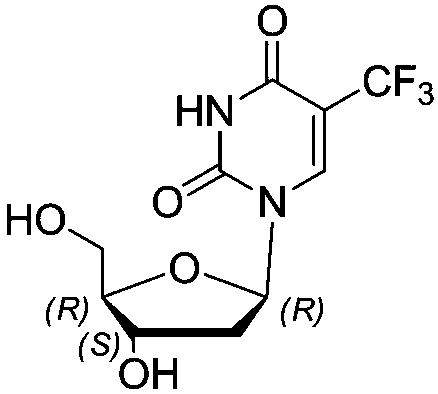
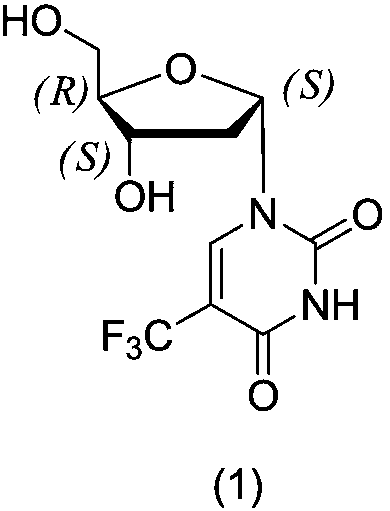
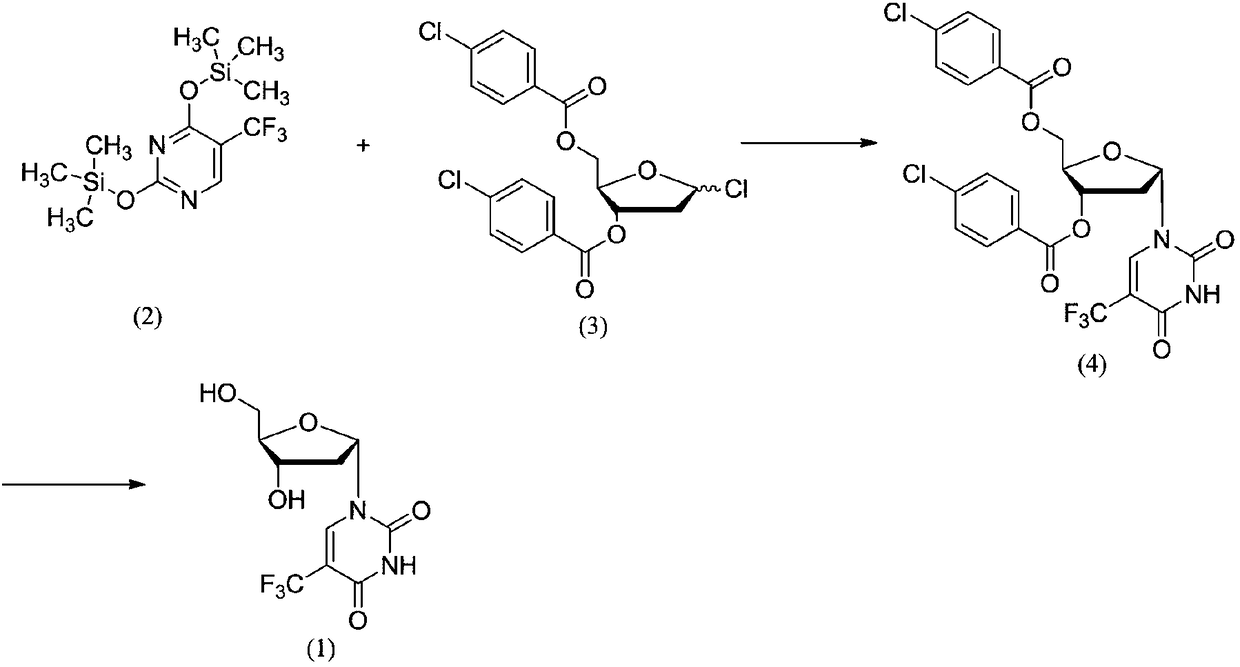
Method for synthesizing trifluridine process impurity
A technology for trifluridine and process impurities, which is applied in the field of chemical pharmacy to achieve the effects of cheap raw materials, improved accurate positioning and characterization, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of 3',5'-bis(p-chlorobenzoyl)-2'-deoxy-α-D-ribose-5-trifluoromethyluracil (4)
[0017]
[0018] Add 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)uracil (15.76g, 0.048mol), 100ml of chloroform, 1-chloro-2-deoxy-3,5 -Di-p-chlorobenzoyl-D-ribose (10.5g, 0.024mol) was stirred at room temperature, reacted for 4h, and detected by TLC, raw material 1-chloro-2-deoxy-3,5-di-p-chlorobenzoyl- D-ribose disappears, and the reaction ends. Rotary evaporate to dryness, add ethanol and anisole, stir, filter and dry with suction. β / α=75:25 was detected by HPLC. The sample was prepared, put on the column, and eluted with dichloromethane-methanol (80:1) to obtain 2.48 g of white solid with a yield of 18.51%.
Embodiment 2
[0019] Example 2: Preparation of 3',5'-bis(p-chlorobenzoyl)-2'-deoxy-α-D-ribose-5-trifluoromethyluracil (4)
[0020] Add 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)uracil (15.76g, 0.048mol), anisole 100ml, 1-chloro-2-deoxy-3 , 5-di-p-chlorobenzoyl-D-ribose (10.5g, 0.024mol) was stirred at 60°C, reacted for 12h, and detected by TLC, raw material 1-chloro-2-deoxy-3,5-di-chlorobenzene Formyl-D-ribose disappears and the reaction ends. Add ethanol and stir, filter and dry with suction. β / α=45:55 was detected by HPLC. The sample was prepared, loaded on the column, and eluted with dichloromethane-methanol (80:1) to obtain 6.91 g of white solid with a yield of 50.27%.
[0021] 1 HNMR (600MHz, DMSO-d6, δppm): 2.33(t, 2H), 3.62(m, 2H), 3.73(t, 1H), 5.31(t, 2H), 7.60(d, 4H), 7.89(m, 4H), 8.57(s, 1H), 11.82(s, 1H).
Embodiment 3
[0022] Example 3: 1-((2S,4S,5R)-4-hydroxyl-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H )-diketone (1) preparation
[0023]
[0024] Add 3',5'-bis(p-chlorobenzoyl)-2'-deoxy-α-D-ribose-5-trifluoromethyluracil (10.3g, 0.018mol) and 130ml methanol to a 200ml three-necked flask Stir in an ice bath, add sodium methoxide (1.94 g, 0.036 mol), react for 4 h, and TLC detects that the reaction is complete. HCl gas was introduced to adjust the pH to 5-6, filtered with suction, and the solvent was rotary evaporated to dryness to obtain 4.82 g of a white solid with a yield of 90.42%. HPLC detection 98.27%.
[0025] 1 HNMR (600MHz, DMSO-d6, δppm): 2.31(t, 2H), 3.64(m, 2H), 3.78(t, 1H), 4.25(s, 1H), 5.29(t, 2H), 6.06(t, 1H), 8.62(s, 1H), 11.84(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com