Improved method of preparation of trifluridine
A technology for trifluoromethylation and compound, applied in the field of preparation of "trifluridine, can solve the problems of difficult separation of isomers, environmental pollution, high toxicity of xenon difluoride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
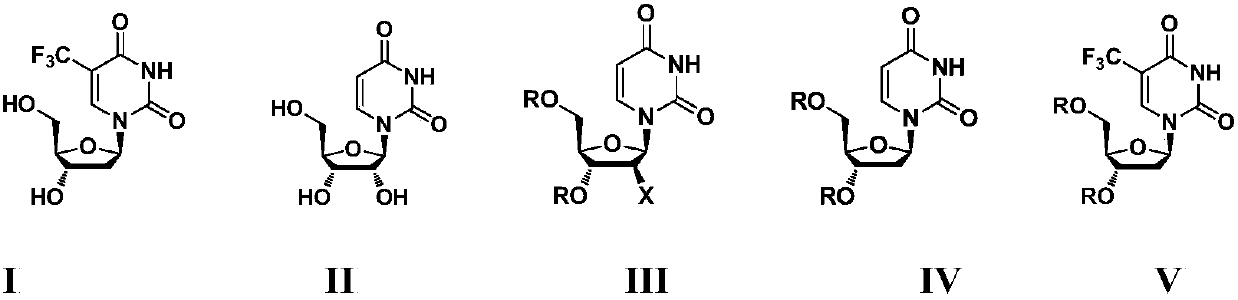
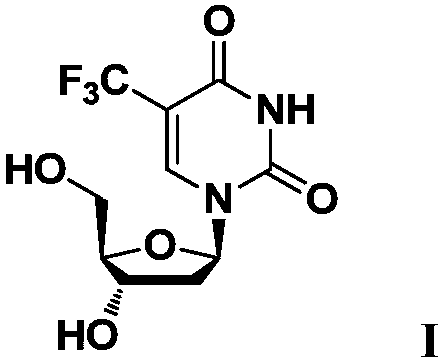
[0046] Preparation of "trifluridine" (compound shown in formula I)
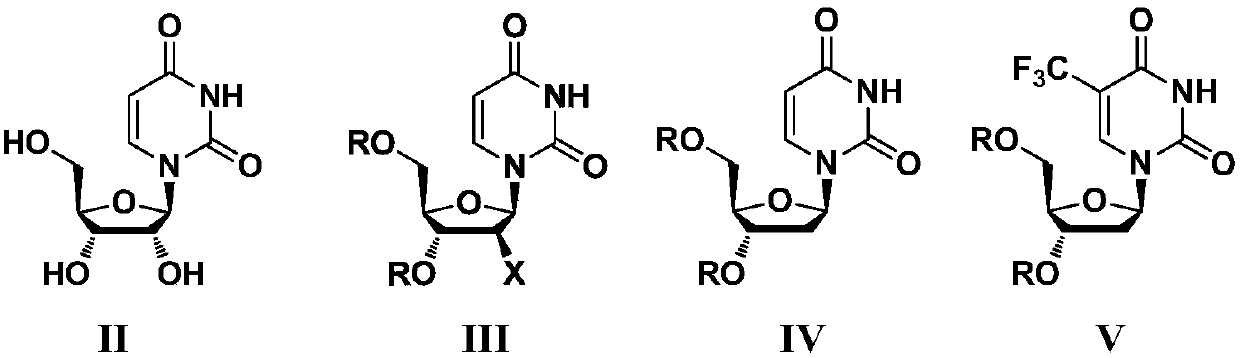
[0047] (1) Preparation of compound shown in intermediate formula IIIa or IIIb:
[0048]
[0049] Add 600mL of tetrahydrofuran, 30.0g of the compound shown in formula II and 68mL of triethylamine into a 1000mL three-necked flask, stir and raise the temperature to reflux, under reflux conditions, add 27mL of acetyl chloride dropwise within 30min, then reflux for 3h, cool to room temperature and spin until bubbling, add 600mL dichloromethane and wash twice with 200mL ice water, 200mL saturated NaHCO 3 The aqueous solution was washed once, dried over anhydrous sodium sulfate, and spin-dried to obtain 41.7 g of the compound represented by formula IIIa, with a yield of 98%; or,
[0050] Add 600mL of tetrahydrofuran, 30.0g of the compound represented by formula II and 68mL of triethylamine into a 1000mL three-necked flask, stir and heat up to 65-70°C, add 33mL of acetyl bromide dropwise within 30min, react at 65...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com