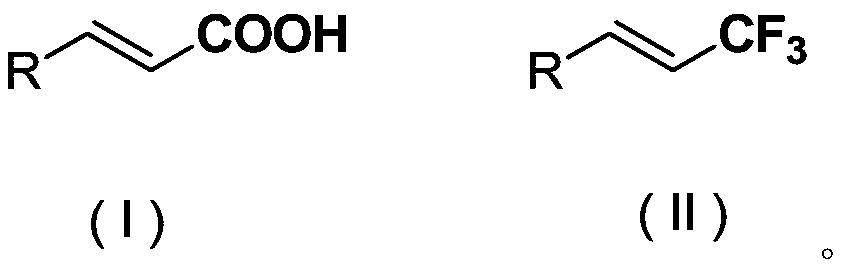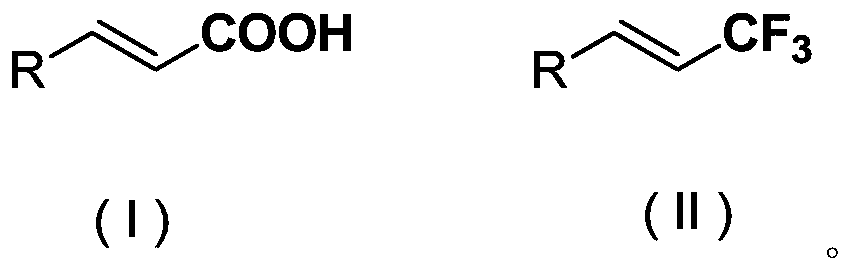Method for synthesizing compounds containing vinyl trifluoromethyl structure
A technology of vinyl trifluoromethyl and trifluoromethyl sources is applied in electrolytic process, electrolytic components, electrolytic organic production, etc., to achieve the effects of low price, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
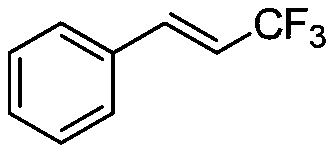
[0024] The synthesis of embodiment 1 (E)-(3,3,3-trifluoroprop-1-en-1-yl)benzene
[0025]
[0026] Take a three-neck round bottom flask, add 5 mL of dimethyl sulfoxide and 2.5 mL of dichloromethane, 74 mg (0.50 mmol) of cinnamic acid, 234 mg (1.5 mmol) of sodium trifluoromethylsulfinate, and 97 mg of tetra-n-butylammonium hexafluorophosphate (0.25mmol), with a carbon rod as the anode and a platinum sheet as the cathode, the electrochemical reaction was stirred at room temperature for 3 hours under a current of 7mA. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, and 5 mL of saturated brine was added for washing. After separation, the organic phase was collected, and the aqueous phase was extracted 3 times with ethyl acetate, each time the amount of ethyl acetate was 5 mL, and the organic phase was combined. Anhydrous sodium sulfate was added for drying, the solvent was distilled off under reduced pressure, and the product was obtain...
Embodiment 2
[0028] Synthesis of Example 2 (E)-1-methyl-4-(3,3,3-trifluoroprop-1-en-1-yl)benzene
[0029]
[0030] Take a three-neck round bottom flask, add 5 mL of dimethyl sulfoxide, 2.5 mL of dichloromethane, 81 mg (0.50 mmol) of (E)-3-(p-tolyl)acrylic acid, and 234 mg (1.5 mmol) of sodium trifluoromethylsulfinate . 97 mg (0.25 mmol) of tetra-n-butylammonium hexafluorophosphate, with a carbon rod as an anode and a platinum sheet as a cathode, and electrochemically reacted at room temperature for 3 hours under stirring at a current of 7 mA. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, and 5 mL of saturated brine was added for washing. After separation, the organic phase was collected, and the aqueous phase was extracted 3 times with ethyl acetate, each time the amount of ethyl acetate was 5 mL, and the organic phase was combined. Anhydrous sodium sulfate was added for drying, the solvent was distilled off under reduced pressure, and the pr...
Embodiment 3
[0032] Synthesis of Example 3 (E)-1-methoxy-4-(3,3,3-trifluoroprop-1-en-1-yl)benzene
[0033]
[0034] Take a three-neck round bottom flask, add 5 mL of dimethyl sulfoxide, 2.5 mL of dichloromethane, 89 mg (0.50 mmol) of (E)-3-(4-methoxyphenyl) acrylic acid, and 234 mg of sodium trifluoromethylsulfinate (1.5mmol), 97mg (0.25mmol) of tetra-n-butylammonium hexafluorophosphate, with a carbon rod as the anode and a platinum sheet as the cathode, and stirred at room temperature for an electrochemical reaction at 7mA for 3 hours. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, and 5 mL of saturated brine was added for washing. After separation, the organic phase was collected, and the aqueous phase was extracted 3 times with ethyl acetate, each time the amount of ethyl acetate was 5 mL, and the organic phase was combined. Anhydrous sodium sulfate was added to dry, the solvent was distilled off under reduced pressure, and the product was ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com