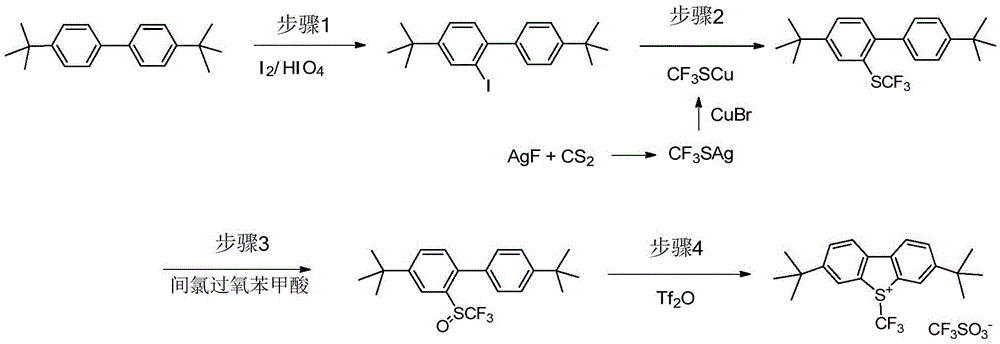
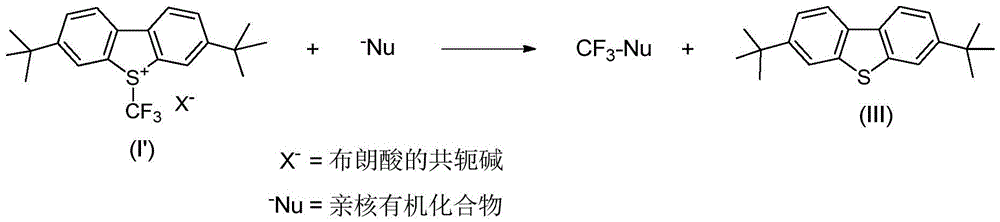
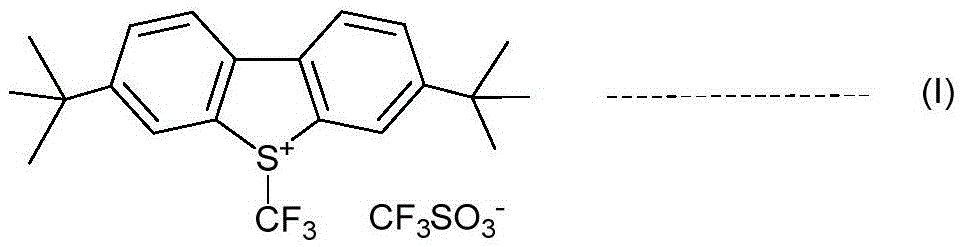
An industrial production method for 3,7-bis(tertiary butyl)-S-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate
A technology of benzothiophene trifluoromethanesulfonate and trifluoromethylsulfinic acid, applied in 3,7-di-tert-butyl-S-(trifluoromethyl)dibenzothiophene trifluoromethanesulfonate In the field of industrial production of acid salts, it can solve problems such as unsuitable for industrial production, major environmental problems, and low production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
[0075] Dry sodium trifluoromethylsulfinate (6.44 g, 41.3 mmol), 4,4'-di-tert-butylbiphenyl (5.0 g, 18.8 mmol), and 25 ml of dry nitromethane were added to a round bottom flask. Nitrogen replacement, after cooling in an ice bath, trifluoromethanesulfonic anhydride (12.7 g, 45.0 mmol) was added dropwise for 20 minutes. The ice bath was removed, and the reaction solution was gradually warmed to room temperature and stirred at room temperature for 19 hours. With benzotrifluoride as internal standard, 19 The reaction solution was detected by FNMR, and the product 3,7-di-tert-butyl-S-(trifluoromethyl)dibenzothiophene trifluoromethanesulfonate was generated with a yield of 87%. The solvent was evaporated to dryness, 25 ml of toluene was added, and the solvent was evaporated to dryness again. This operation was repeated three times to exhaust the nitromethane. Add 35ml of water and 35ml of ether, and stir for 1 hour. The precipitated solid was filtered to obtain 8.3 g ...
Embodiment 2
[0081]
[0082] Add dry sodium trifluoromethanesulfinate (7.61 g, 48.4 mmol), 4,4'-di-tert-butylbiphenyl (10.0 g, 37.5 mmol), and 50 ml of dry nitromethane into a round bottom flask. Nitrogen replacement, after cooling in an ice bath, trifluoromethanesulfonic anhydride (15.1g, 53.6mmol) was added dropwise, and the dripping was completed in 20 minutes, then stirred for 10 minutes, the ice bath was removed, and trifluoroacetic anhydride (8.66g, 41.3mmol) was added in one go. mmol), the reaction solution was stirred at room temperature for 16 hours. With benzotrifluoride as internal standard, 19 The reaction solution was detected by FNMR, and the product 3,7-di-tert-butyl-S-(trifluoromethyl)dibenzothiophene trifluoromethanesulfonate was generated with a yield of 40%. The solvent was evaporated to dryness, a certain amount of toluene was added, and the solvent was evaporated to dryness again. Add 25ml of water and stir for 10 minutes. Filtration yielded 9.88 g of solid, whic...
Embodiment 3
[0084]
[0085] Dry sodium trifluoromethylsulfinate (3.8 g, 24.4 mmol), 4,4'-di-tert-butylbiphenyl (5.0 g, 18.8 mmol), and 25 ml of dry nitromethane were added to a round bottom flask. Nitrogen replacement, after cooling in an ice bath, add trifluoroacetic anhydride (9.9 g, 47.0 mmol) dropwise for 10 minutes, then stir for another 10 minutes, stir the reaction solution for 1.5 hours, slowly add trifluoromethanesulfonic anhydride to maintain the internal temperature The temperature was not higher than 25°C, and the addition was completed in 15 minutes, then stirred in an ice bath for 30 minutes, then removed the ice bath, and the reaction solution was stirred at room temperature for 22 hours. With benzotrifluoride as internal standard, 19The reaction solution was detected by FNMR, and the product 3,7-di-tert-butyl-S-(trifluoromethyl)dibenzothiophene trifluoromethanesulfonate was generated with a yield of 51%. The solvent was evaporated to dryness, 25ml of toluene was added,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com