Electrochemical synthesis method of trifluoromethylated aryl amide derivative
A technology of trifluoromethylated arylamide and fluoromethylated arylamide, which is applied in the field of electrochemical synthesis of trifluoromethylated arylamide derivatives, can solve the problems of non-compliance with green chemistry, and reach the bottom line wide applicability, mild reaction conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
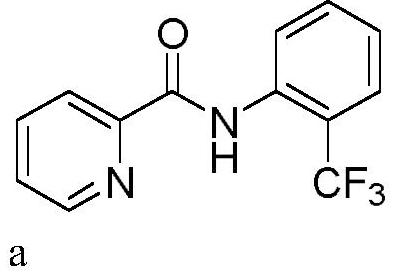
[0028] In a 10mL three-neck flask equipped with a carbon anode (d=6mm) and a platinum plate (1cm x 1cm) cathode, add N-phenylpyridinamide (0.3mmol, 59.5mg), sodium trifluoromethylsulfinate (0.45mmol , 70.2mg), tetrabutylammonium bromide (0.3eq, 29.0mg). Acetonitrile (3.0 mL) was added. The reaction mixture was stirred at 50° C. for 120 min at a constant current of 15 mA. After the reaction, TLC detection, the reaction mixture was concentrated under reduced pressure. Purification was carried out by silica gel column chromatography to obtain 54.3 mg of the target product a as a white solid, with a yield of 68%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ10.60(s,1H),8.60(d,J=4.7Hz,1H),8.53(d,J=8.3Hz,1H),8.24(d,J=7.8Hz,1H),7.86(td, J=7.7,1.7Hz,1H),7.61(d,J=7.7Hz,1H),7.55(t,J=7.7Hz,1H),7.44(ddd,J=7.6,4.8,1.1Hz,1H), 7.18(d,J=7.7Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ161.18, 148.09, 147.08, 136.42, 134.28 (q, J=2.5Hz), 131.69, 126.15 (q, J=5.0Hz), 125.48, 123.98 (q, J=273.4Hz...
Embodiment 2
[0031]
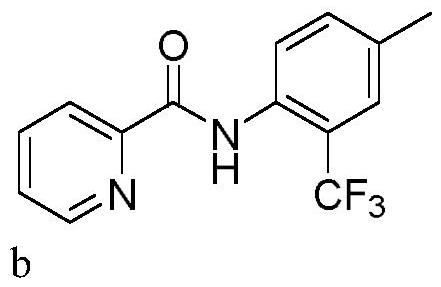
[0032] In a 10mL three-neck flask equipped with a carbon anode (d=6mm) and a platinum plate (1cm x 1cm) cathode, add N-(p-tolyl)pyridinamide (0.3mmol, 63.7mg), sodium trifluoromethylsulfinate (0.45mmol, 70.2mg), tetrabutylammonium bromide (0.3eq, 29.0mg). Acetonitrile (3.0 mL) was added. The reaction mixture was stirred at 50° C. for 120 min at a constant current of 15 mA. After the reaction, TLC detection, the reaction mixture was concentrated under reduced pressure. Purification was carried out by silica gel column chromatography to obtain 54.6 mg of the target product b as a white solid, with a yield of 65%.
[0033] 1 H NMR (400MHz, CDCl 3 )δ10.57(s,1H),8.68(d,J=4.4Hz,1H),8.43(d,J=8.4Hz,1H),8.31(d,J=7.8Hz,1H),7.93(td, J=7.7,1.7Hz,1H),7.52(ddd,J=7.6,4.8,1.1Hz,1H),7.48(s,1H),7.43(d,J=8.4Hz,1H),2.42(s,3H ). 13 C NMR (100MHz, CDCl 3)δ162.36, 149.46, 148.29, 137.66, 134.09, 133.41(q, J=1.3Hz),, 132.89(q, J=1.3Hz), 126.64, 126.55(q, J=5.0Hz), 124.43(q, J= 27...
Embodiment 3
[0035]
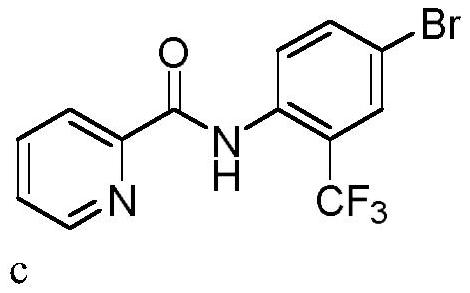
[0036] In a 10mL three-necked flask equipped with a carbon anode (d=6mm) and a platinum plate (1cm x 1cm) cathode, add N-(4-bromophenyl)pyridineamide (0.3mmol, 83.2mg), trifluoromethylsulfin Sodium Acetate (0.45mmol, 70.2mg), Tetrabutylammonium Bromide (0.3eq, 29.0mg). Acetonitrile (3.0 mL) was added. The reaction mixture was stirred at 50° C. for 120 min at a constant current of 15 mA. After the reaction, TLC detection, the reaction mixture was concentrated under reduced pressure. Purification was carried out by silica gel column chromatography to obtain 62.1 mg of the target product c as a white solid, with a yield of 60%.
[0037] 1 H NMR (400MHz, CDCl 3 )δ10.69(s,1H),8.67(d,J=4.2Hz,1H),8.57(d,J=8.9Hz,1H),8.29(d,J=7.8Hz,1H),7.93(td, J=7.7,1.6Hz,1H),7.79(d,J=1.9Hz,1H),7.73(d,J=8.9Hz,1H),7.53(ddd,J=7.6,4.8,1.1Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ161.35, 148.05, 147.34, 136.72, 134.86, 133.75(q, J=1.0Hz), 130.14(q, J=5.0Hz), 125.87, 123.34, 122.18(q, J=274.2Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com