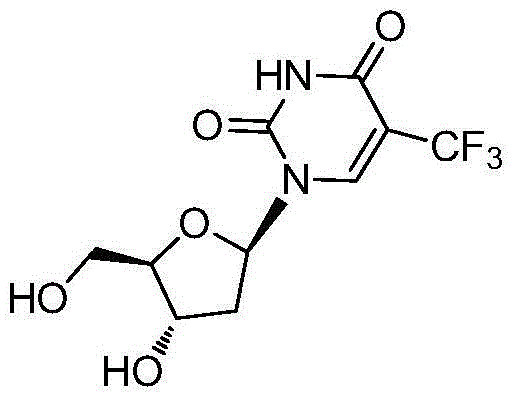
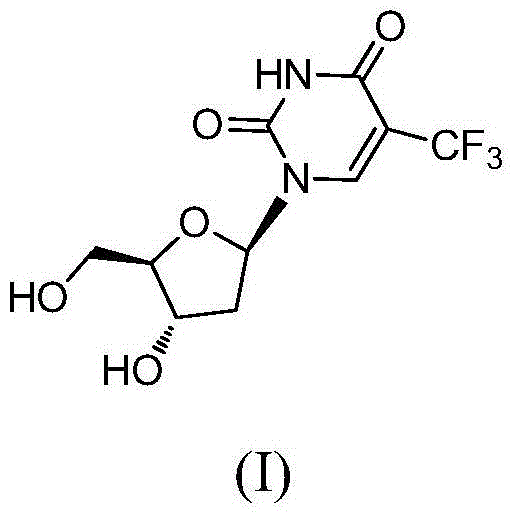
Novel trifluridine crystal form and preparation method thereof
A technology of trifluridine crystal and crystal form, which is applied in the field of drug preparation, can solve the problems of no crystal form patent report of trifluridine, and achieve the effect of stable crystal form properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
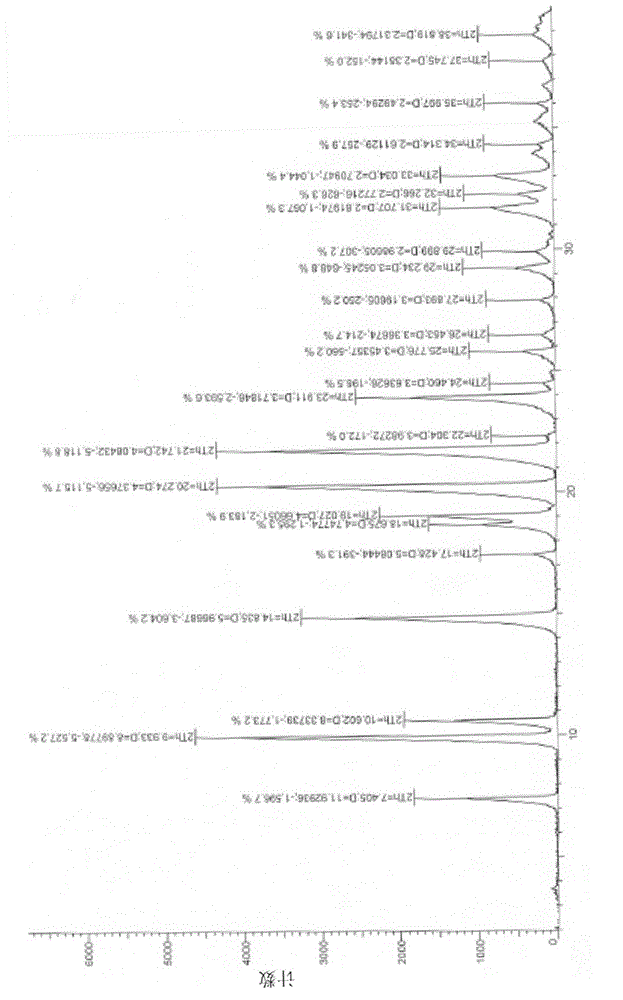
[0028] Weigh 10g of crude trifluridine, add it to 90ml of methanol, dissolve at 10°C, slowly add 2700ml of dichloromethane dropwise, stir at 10°C for 12h, filter to obtain 7.1g of white solid, which is determined to be crystal form II by X-ray diffractometer , whose spectrum is as figure 1 shown.
Embodiment 2
[0030] Weigh 10g of crude trifluridine, add it to 100ml of ethanol, dissolve at 10°C, slowly add 3000ml of dichloromethane dropwise, stir at 10°C for 12h, filter to obtain 7.7g of white solid, which is determined to be crystal form II by X-ray diffractometer .
Embodiment 3
[0032] Weigh 10g of crude trifluridine, add it to 70ml of methanol, heat to reflux to dissolve, turn off the heating, cool down to 10°C and stir for 12h, filter to obtain 5.3g of white solid, which is determined to be crystal form II by X-ray diffractometer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com