Application of 6 Small Molecule Drugs in Inhibiting Canine Parvovirus
A technology of canine parvovirus and medicine, applied in the field of known compounds, can solve the problems of vaccine weakening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
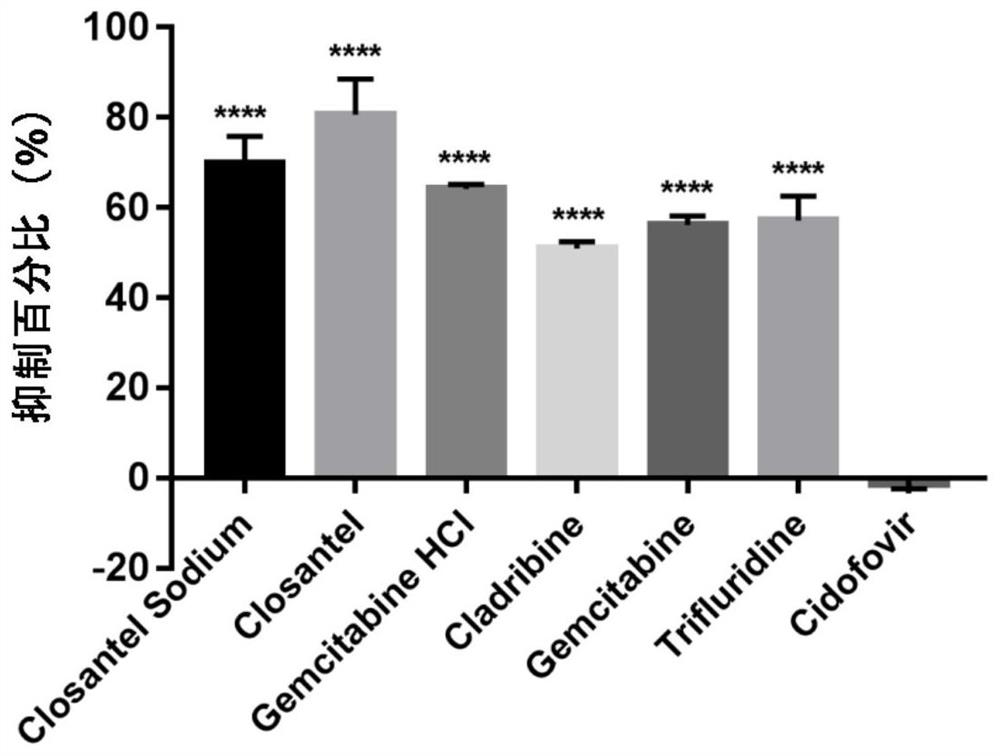
[0061] Example 1. Determination of the protective effect of 6 small molecule drugs on CPV inhibition in F81 cells
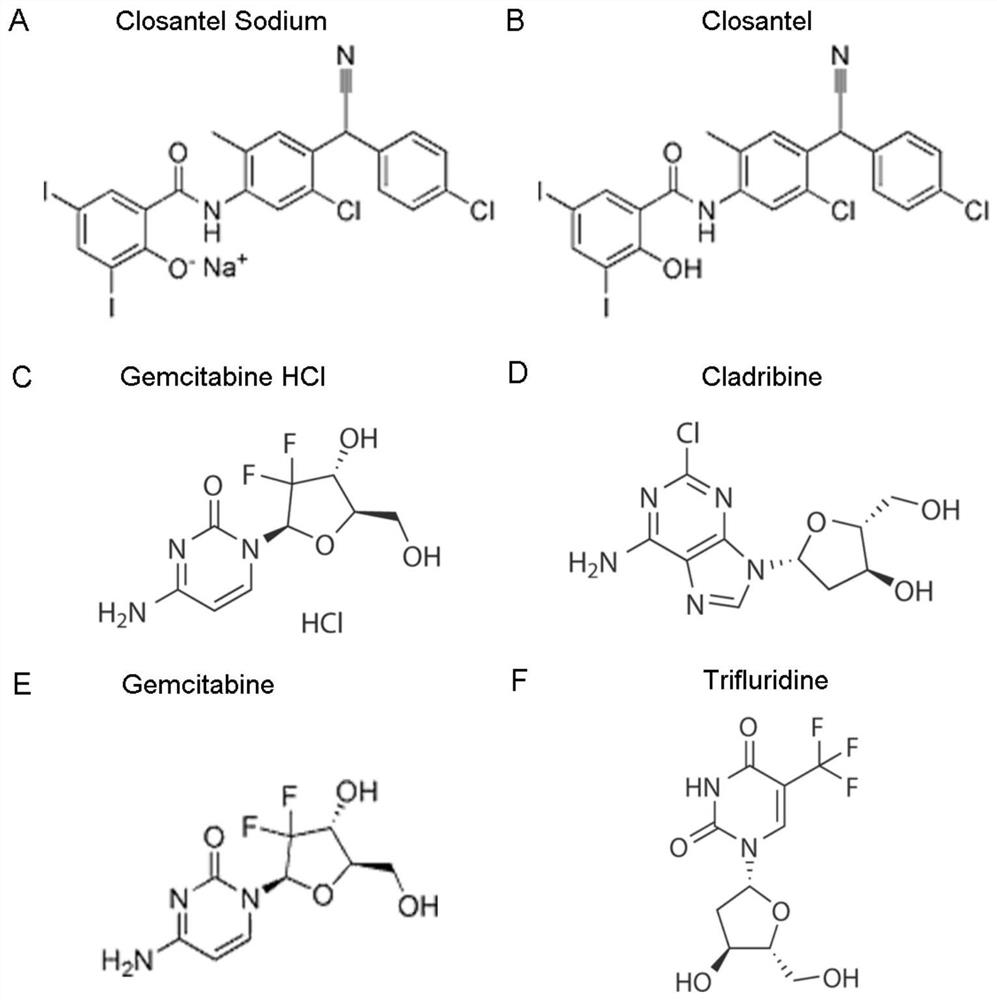
[0062] Before F81 cells were purchased and used, 4 μL of small molecule drugs (Closantel / Closantel Sodium / Gemcitabine HCl / Trifuridine / Gemcitabine / Cladribine) (10 mM) and 4 μL of control drug Cidofovir (10 mM) were added to 156 μL of maintenance medium (MM), respectively, A 250 μM drug stock solution was prepared.
[0063]Drug treatment group: each drug stock solution was used to treat the F81 cells respectively. For each treatment group, 4 μL of 250 μM drug stock solution was added to 86 μL of F81 cells (25,000 cells per well), and the final concentration of each drug was 10 μM. After continuous treatment for 1 hour, 10 μL of CPV (New CPV-2a strain SD6) The drug-treated F81 cells were infected with an MOI (multiplicity of infection) of 0.076. Cell viability was assayed 40 hours after infection.
[0064] Positive control: DMSO was added to F81 cells to a final ...
Embodiment 2
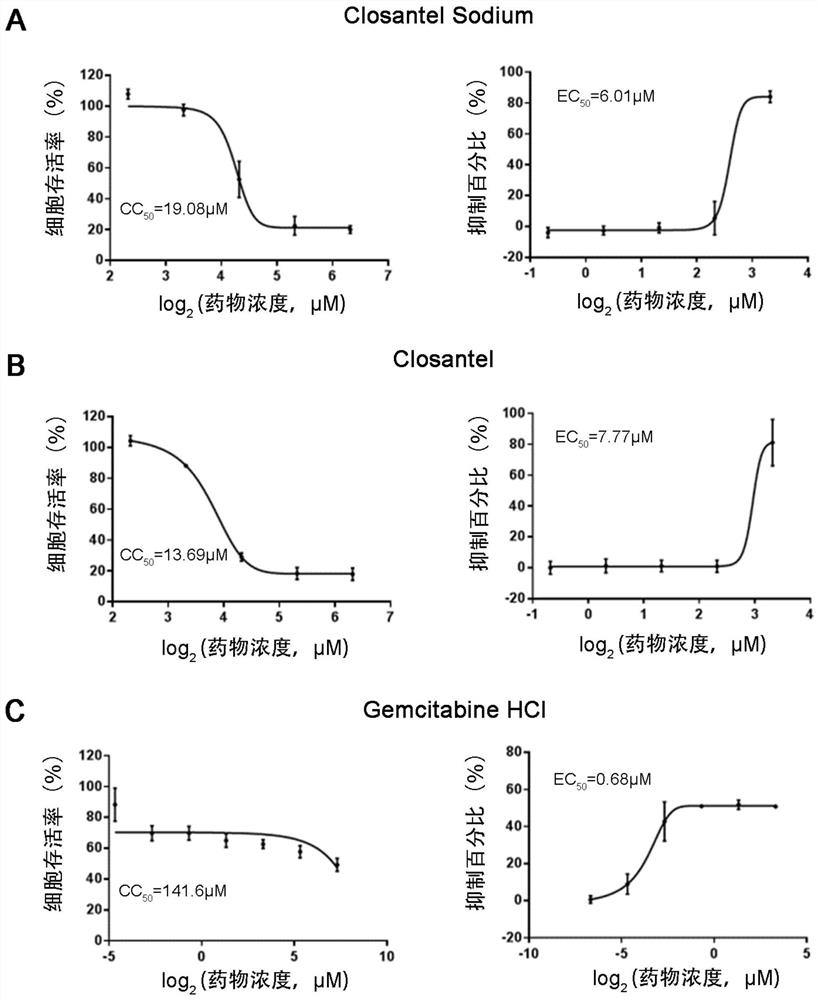
[0069] Example 2. Determination of 50% effect concentration (50% antiviral efficacy, EC50) and 50% cytotoxicity concentration (50% cytotoxicity concentrations, CC50) of 6 kinds of small molecule drugs
[0070] The EC50 and CC50 of the drugs were determined by dose-response assay. details as follows:
[0071] The EC50 determination procedure is as follows: 86 μL of F81 cells (25,000 cells per well) were pretreated with 4 μL of doubly diluted drug (Closantel / Closantel Sodium / Gemcitabine HCl / Trifuridine / Gemcitabine / Cladribine) (final concentration range 0.3125-20 μM) for 1 h , and then 10 μL of CPV (New CPV-2a strain SD6) (MOI=0.076) was used to infect the drug-treated cells.
[0072] The CC50 determination procedure is as follows: 96 μL of F81 cells (25,000 cells per well) are mixed with 4 μL of doubly diluted drug (final concentration range is 0.3125-80 μM).
[0073] Both EC50 and CC50 determinations were performed in triplicate. After 40 hours of incubation, the Cell Coun...
Embodiment 3
[0077] Example 3. Immunofluorescence assay (IFA) detection of inhibition of viral VP2 protein expression by six small molecule drugs
[0078] Drug treatment group: 86 μL of F81 cells in a 96-well plate (25,000 cells per well) were mixed with 4 μL of doubly diluted drugs (Closantel / Closantel Sodium / Gemcitabine HCl / Trifluridine / Gemcitabine / Cladribine) (the final concentration of the drug was 5 μM, respectively, 10 μM and 20 μM) were pretreated for 1 hour, and the treated cells were infected with 10 μL CPV (New CPV-2a strain SD6) (MOI=0.076). Immunofluorescence assays were performed approximately 30 hours after infection.
[0079] Control group: DMSO was added to F81 cells in a 96-well plate to a final concentration of 0.1% (volume fraction), and an immunofluorescence test was performed 30 hours later.
[0080] Immunofluorescence test:
[0081] Fixation: Discard the culture medium in the 96-well plate and wash with 1×PBS (Gibco TM , USA, article number: 20012050) wash 3 times,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com