Closantel or its sodium salt long-acting sustained-release injection and preparation thereof
A technology of iodoclosamide and sustained-release injections, which is applied in the direction of pharmaceutical formulations, nitrile/isonitrile active ingredients, oil/fat/wax non-active ingredients, etc., and can solve the problem of lack of long-term anti-parasitic activity and drug effect time Short and other problems, to achieve the effect of convenient storage and transportation, good stability and stable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
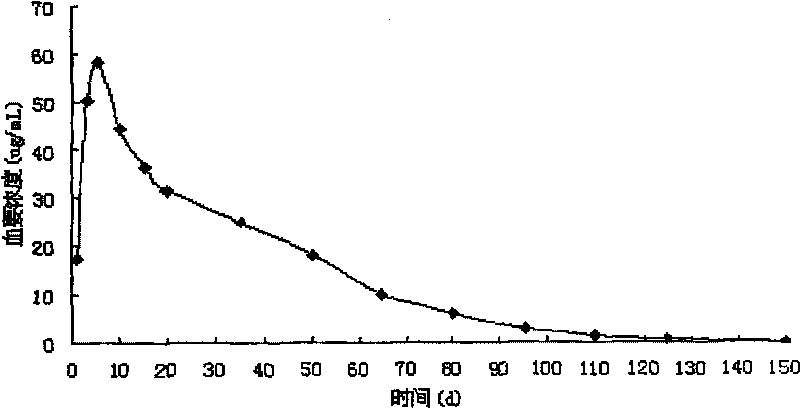
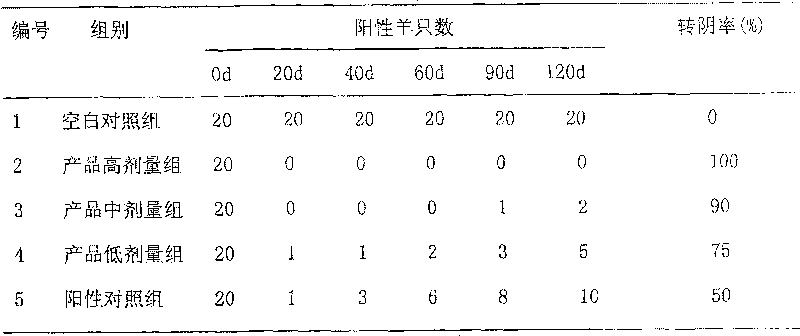
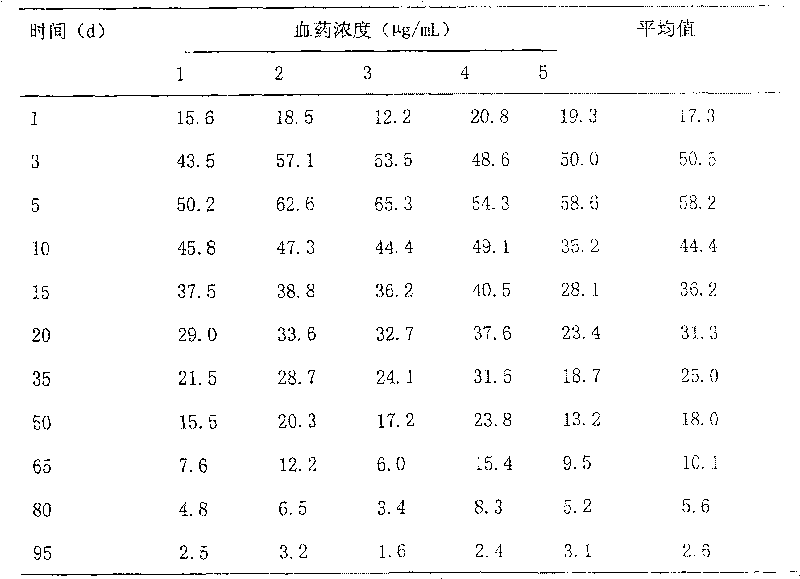
Image
Examples
Embodiment 1
[0019] 1) Put 80 parts of castor oil for injection into a beaker and heat it to 50°C; then weigh 2 parts of aluminum monostearate into the castor oil, stir to form a white uniform paste, and swell for 10 hours. Heat the swollen white pasty mixture to 130°C while stirring to make it completely transparent, let it cool to 50°C, and filter through a vertical melting funnel to obtain light yellow transparent aluminum monostearate castor oil gel.
[0020] 2) Weigh 10 parts of iodosamide crude drug, add 40 parts of absolute ethanol, fully shake to dissolve, and filter with a 0.45 μm microporous membrane. Under sterile conditions, slowly add the filtrate to 80 parts of aluminum monostearate castor oil gelatin at 50°C, and stir while adding to form a light yellow transparent liquid, then heat up to 60°C to evaporate 20 parts of absolute ethanol , and subpackage after cooling to obtain a 10% closantamide long-acting sustained-release injection.
Embodiment 2
[0022] 1) Put 50 parts of castor oil for injection into a beaker and heat it to 50°C; then weigh 2 parts of aluminum stearate into the castor oil, stir to form a white uniform paste, and swell for 10 hours. Heat the swollen white pasty mixture to 130°C while stirring to make it completely transparent, let it cool to 50°C, and filter through a vertical melting funnel to obtain light yellow transparent aluminum stearate castor oil gel.
[0023] 2) Weigh 15 parts of iodosamide crude drug, add 60 parts of absolute ethanol, fully shake to dissolve, and filter with a 0.45 μm microporous membrane. Under sterile conditions, slowly add the filtrate to 50 parts of aluminum stearate castor oil gelatin at 50°C, and stir while adding to form a light yellow transparent liquid, heat up to 60°C to evaporate 10 parts of absolute ethanol, After cooling, sub-package to obtain 15% closantamide long-acting sustained-release injection.
Embodiment 3
[0025] 1) Put 50 parts of castor oil for injection into a beaker and heat it to 50°C; then weigh 1 part of aluminum monostearate into the castor oil, stir to form a white uniform paste, and swell for 10 hours. Heat the swollen white pasty mixture to 140°C while stirring to make it completely transparent, let it cool to 50°C, and filter through a vertical melting funnel to obtain light yellow transparent aluminum monostearate castor oil gel.
[0026] 2) Weigh 10 parts of iodosamide sodium crude drug, add 40 parts of dimethyl sulfoxide and 10 parts of absolute ethanol, fully shake to dissolve, and filter with a 0.45 μm microporous membrane. Under sterile conditions, slowly add the filtrate to 50 parts of aluminum monostearate castor oil gelatin at 50°C, and stir while adding to form a light yellow transparent liquid. % Closantamide Sodium Long-acting Sustained Release Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com