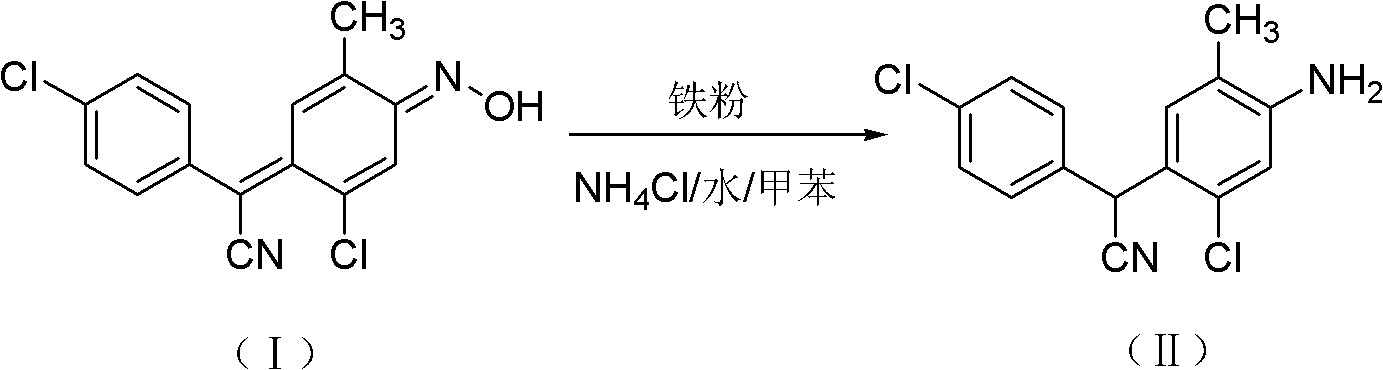
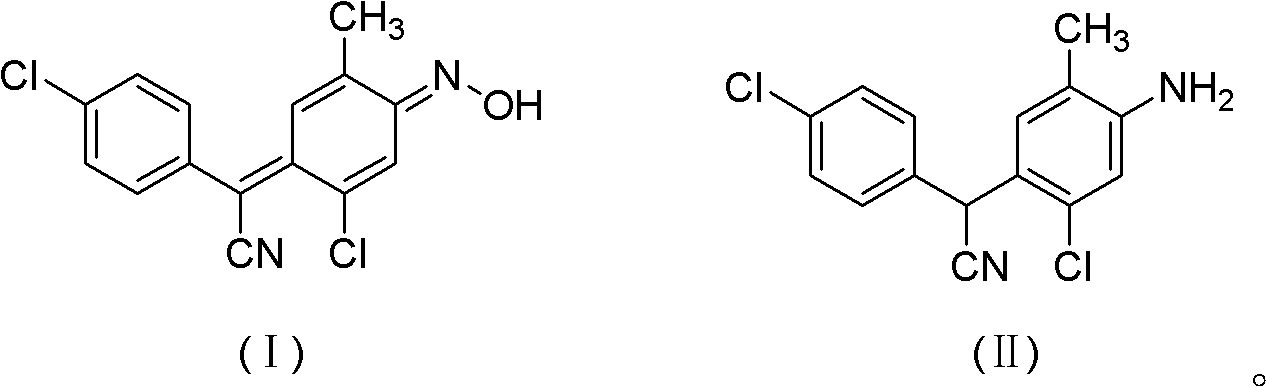
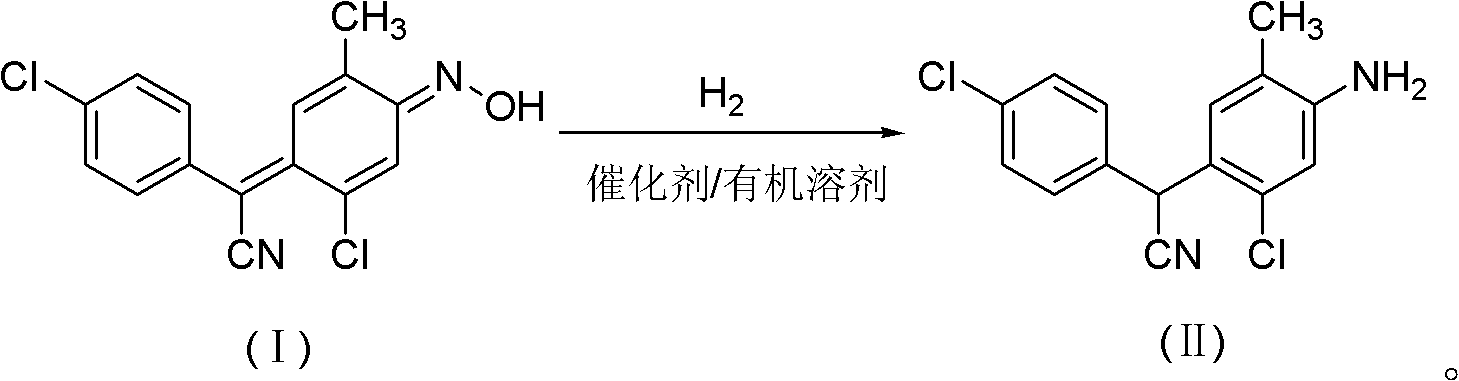
Method for preparing closantel sodium intermediate by catalytic hydrogenation
A technology of closantamide sodium and catalytic hydrogenation, which is applied in the field of medicine and chemical industry, can solve the problems of low product yield and product quality, large amount of raw and auxiliary materials, and relatively large pollution impact, and achieve product yield and purity High, short reaction time, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500mL stainless steel autoclave, add 4-chloro-α-[2-chloro-4-(hydroxyimino)-5-methyl-2,5-cyclohexadienylidene]phenylacetonitrile 30.5g ( 0.1mol) and Ni-Al type nickel 0.3g, then add 200mL of 95% ethanol, replace with hydrogen 3 times, feed hydrogen, control the system pressure to 0.2MPa, start stirring, heat up, control the reaction temperature at 80°C, and react The system pressure drops gradually until the pressure does not drop within 10 minutes, and the reaction takes about 2 hours. After the reaction is finished, after cooling, filter and recycle the catalyst for use. Collect the filtrate, concentrate under reduced pressure to about 40 mL, recover the solvent, and cool the residue to -5°C to 5°C to precipitate crystals, leave it overnight, filter, and dry to obtain the 4-chlorophenyl-(2-chloro-4 -Amino-5-methylphenyl)cyanomethane 27.8 g. The HPLC purity was 97.5%, and the yield was 93.2%.
Embodiment 2
[0022] In a 500mL stainless steel autoclave, add 4-chloro-α-[2-chloro-4-(hydroxyimino)-5-methyl-2,5-cyclohexadienylidene]phenylacetonitrile 30.5g ( 0.1mol) and Ni-Al type nickel 0.3g, then add 200mL of toluene, replace with hydrogen 3 times, feed hydrogen, and control the system pressure to 2MPa, start stirring, raise the temperature, control the reaction temperature at 100°C, the reaction system pressure Decrease gradually until the pressure does not drop within 10 minutes. The reaction takes about 8 hours. After the reaction is completed, after cooling, filter, recover the catalyst and apply it mechanically, collect the filtrate, concentrate under reduced pressure to about 40mL, recover the solvent, and cool the residue to -5℃~ Crystals were precipitated at 5°C, left overnight, filtered, and dried to obtain 27.2 g of 4-chlorophenyl-(2-chloro-4-amino-5-methylphenyl)cyanomethane. The HPLC purity was 98.2%, and the yield was 91.9%.
Embodiment 3
[0024] In a 500mL stainless steel autoclave, add 4-chloro-α-[2-chloro-4-(hydroxyimino)-5-methyl-2,5-cyclohexadienylidene]phenylacetonitrile 30.5g ( 0.1mol) and Ni-Al type nickel 0.03g, then add 300mL of xylene, first pass through hydrogen for replacement 3 times, then pass through hydrogen, and control the system pressure to 5MPa, start stirring, heat up, and control the reaction temperature at 120°C , the pressure of the reaction system drops gradually until the pressure does not drop within 10 minutes, and the reaction takes about 10 hours. After the reaction is completed, cool, filter, recover the catalyst and apply it, collect the filtrate, concentrate under reduced pressure to about 40mL, recover the solvent, cool the residue to -5°C to 5°C to precipitate crystals, leave it overnight, filter, and dry to obtain 4-chlorobenzene 26.3 g of di-(2-chloro-4-amino-5-methylphenyl)cyanomethane, the HPLC purity was 95.6%, and the yield was 86.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com