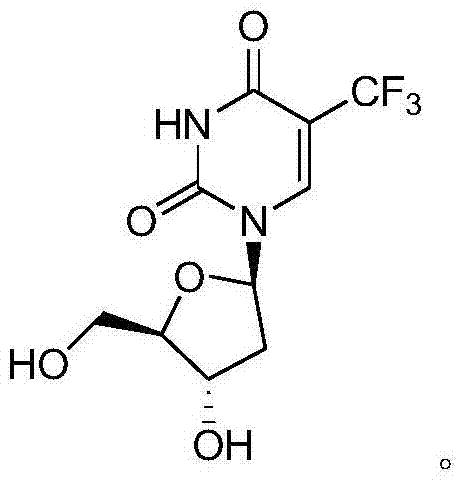
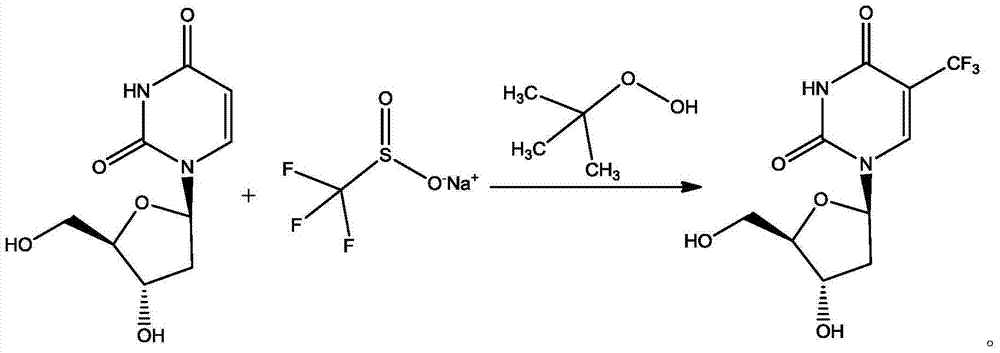
Preparation method of trifluridine
A technology of trifluridine and deoxyuridine, which is applied in the field of preparation and preparation of trifluridine, can solve the problems of low product yield, long reaction route, complicated operation, etc., achieve high purity, short reaction time, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 228g (1mol) of 2'-deoxyuridine and 492.6g (3mol) of 95% sodium trifluoromethanesulfinate into 3.99L of purified water, stir and cool down to -3°C, protect with nitrogen flow, stir to dissolve and add dropwise 386g (3mol) 70% tert-butyl hydroperoxide, the temperature control during the dropwise addition is less than 5°C, after the dropwise addition is completed, heat up to 60°C, stir and react for 2 hours, and the reaction is complete; The ester was extracted 3 times, the extracts were combined, and concentrated under reduced pressure at 50° C. to obtain 276 g of trifluridine, with a yield of 93.2% and a purity of 96.8%.
Embodiment 2
[0035] Add 456g (2mol) of 2'-deoxyuridine and 985.2g (6mol) of 95% sodium trifluoromethanesulfinate into 7.98L of purified water, stir and cool down to -3°C, protect with nitrogen flow, stir to dissolve and add dropwise 772g (6mol) 70% tert-butyl hydroperoxide, temperature control during the dropwise addition is less than 5°C, after the dropwise addition, heat up to 60°C, stir and react for 2 hours, the reaction is complete; drop to room temperature, add 8L ethyl acetate The ester was extracted 3 times, the extracts were combined, and concentrated under reduced pressure at 50° C. to obtain 558 g of trifluridine, with a yield of 94.3% and a purity of 97.2%.
Embodiment 3
[0037] Add 1.14kg (5mol) of 2'-deoxyuridine and 2.46kg (15mol) of 95% sodium trifluoromethanesulfinate into 19.95kg of purified water, stir and cool down to -5°C, protect with nitrogen flow, stir and dissolve, then drop Add 2.12kg (16.5mol) of 70% tert-butyl hydroperoxide, and control the temperature during the dropwise addition to less than 5°C. After the dropwise addition, heat up to 65°C, stir and react for 3 hours, and the reaction is complete; 18 kg of ethyl acetate was extracted three times, the combined extracts were concentrated under reduced pressure at 50° C. to obtain 1.36 kg of trifluridine, yield: 91.9%, purity: 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com