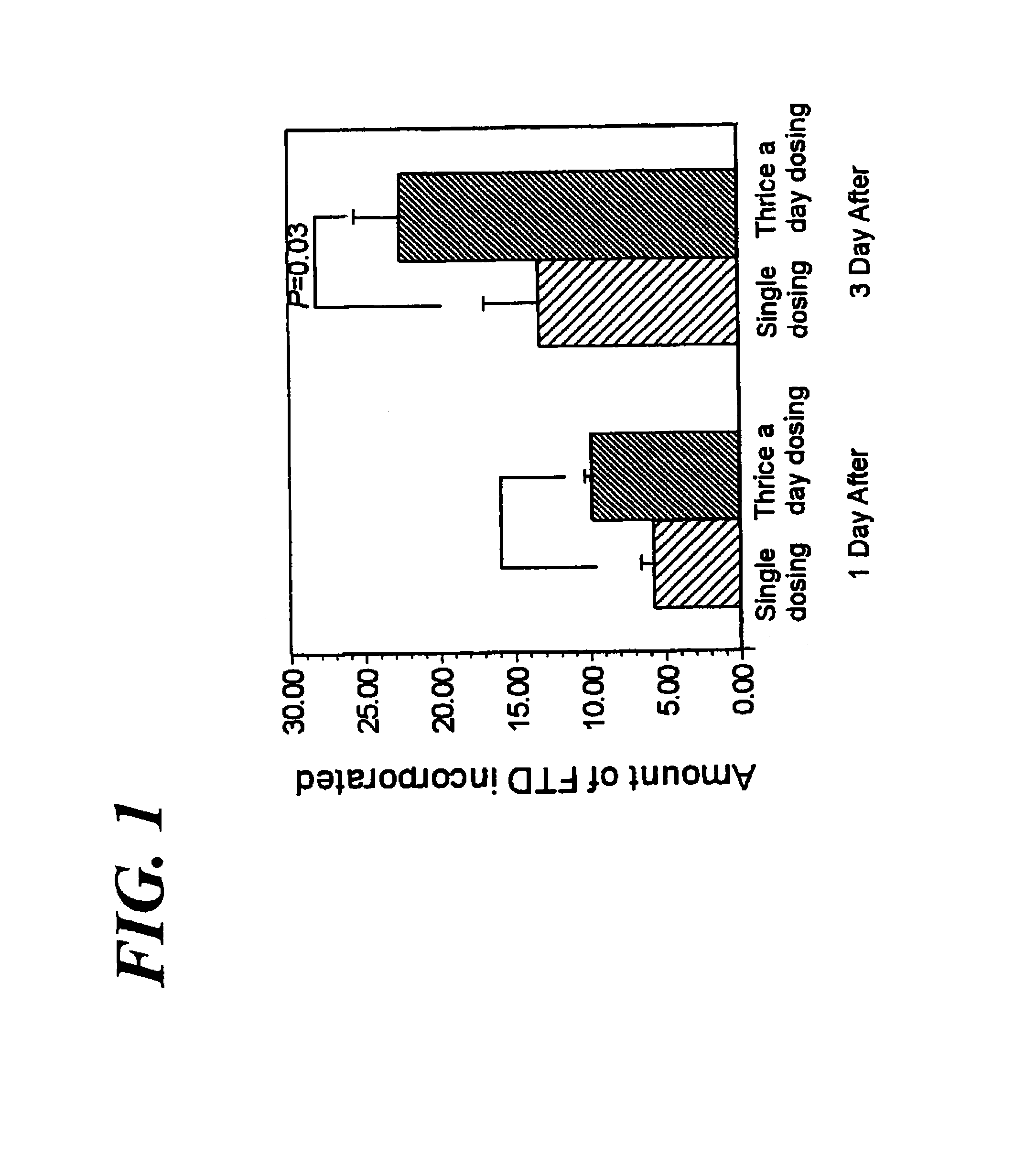
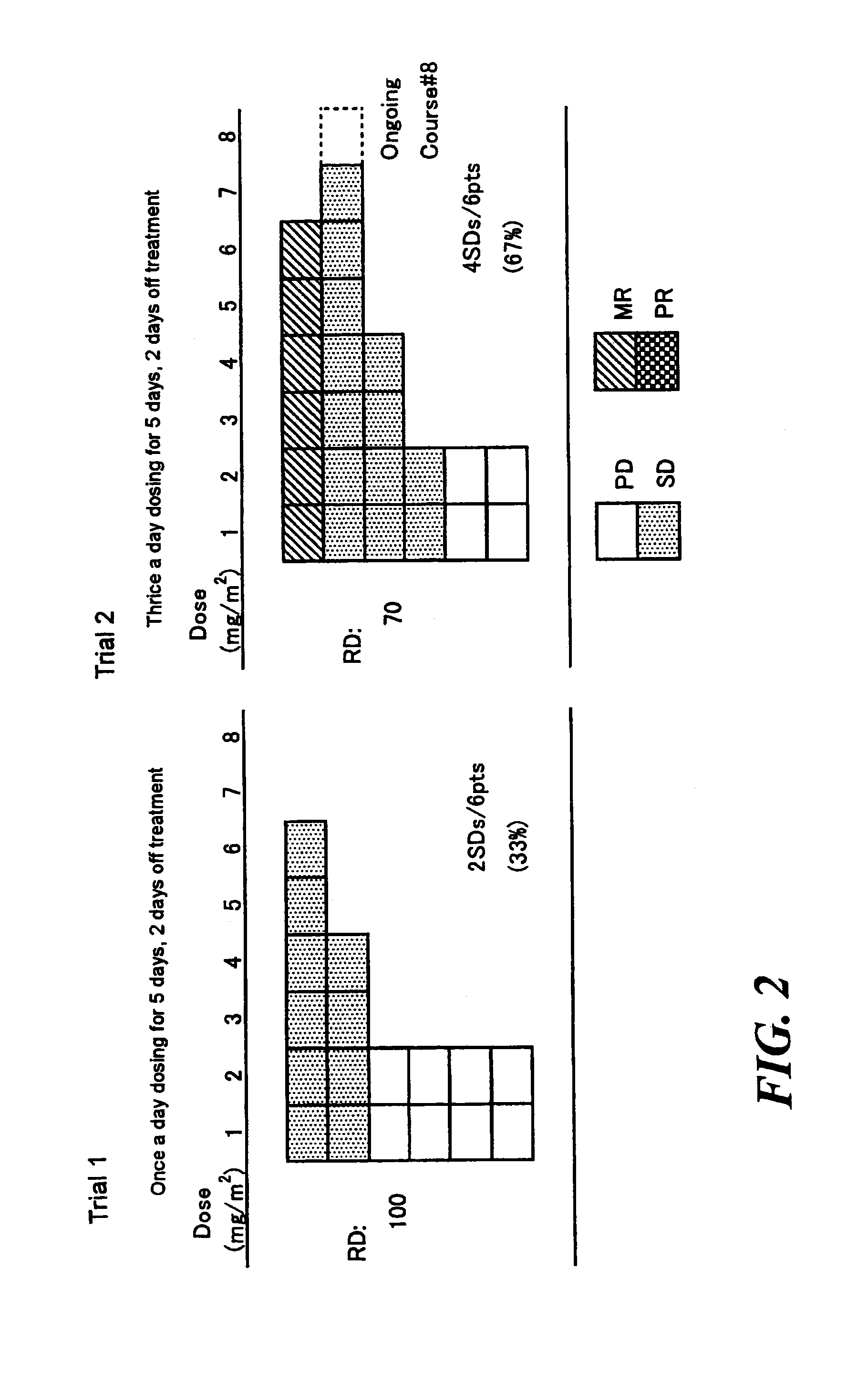
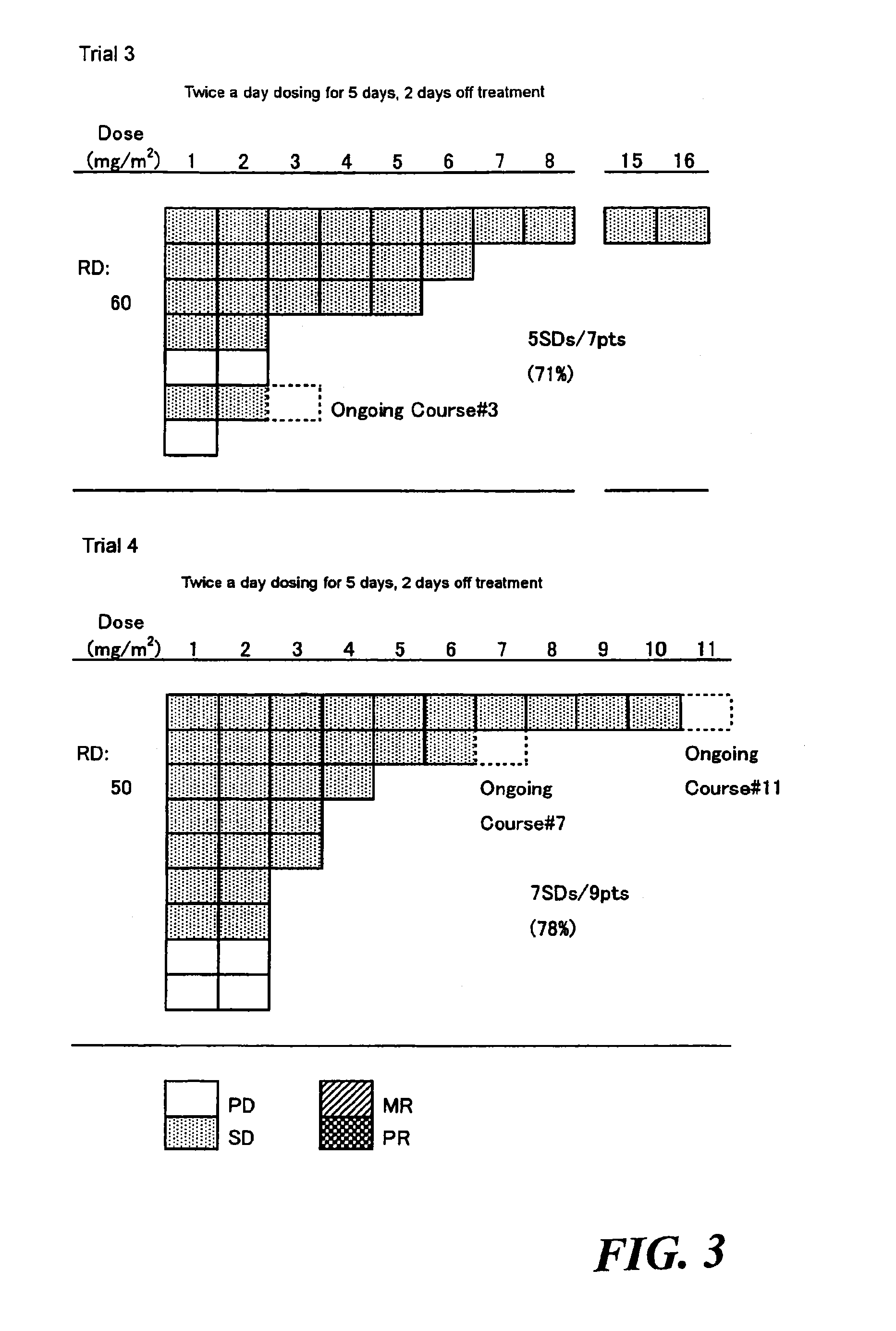
Method of administrating an anticancer drug containing α, α, α-trifluorothymidine and thymidine phosphorylase inhibitor
a technology of trifluorothymidine and phosphorylase inhibitor, which is applied in the direction of drug compositions, medical preparations, antineoplastic agents, etc., can solve the problems of not being able to contribute to the survival period, shortened the half-life of the drug in the blood, and not being able to administer general usage, etc., to achieve a favorable treatment effect against cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0035]
FTD20.00mgTPI-19.42Lactose70.00Crystalline cellulose3.50Magnesium stearate1.00Talc1.00Corn starch3.50Hydroxypropylmethylcellulose25.00Per tablet133.42mg
[0036]A tablet was prepared in the preceding compounding ratio according to the ordinary method.
preparation example 2
[0037]
FTD15.00mgTPI-17.07Lactose45.00Carboxymethylcellulose5.00Magnesium stearate2.00Titanium oxide0.50Hydroxypropylmethylcellulose1.00Polyethylene glycol 40000.50per tablet85.07mg
[0038]A tablet was prepared in the preceding compounding ratio according to the ordinary method.
preparation example 3
[0039]
FTD30.00mgTPI-114.13Lactose85.00Corn starch100.00Hydroxypropylcellulose2.50Per divided dose of powders231.63mg
[0040]A granule was prepared in the preceding compounding ratio according to the ordinary method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com