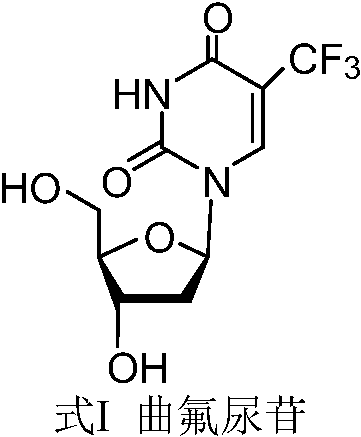
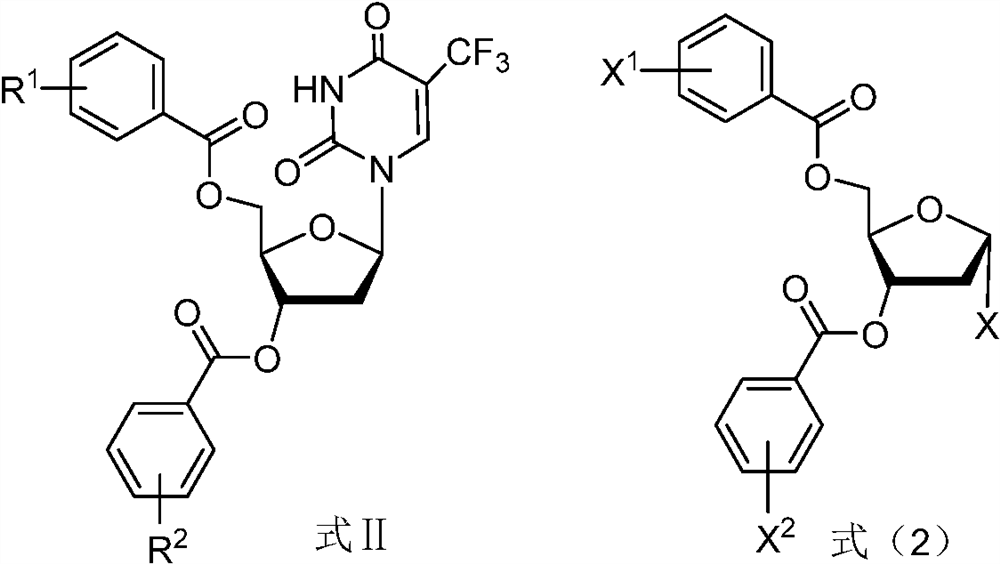
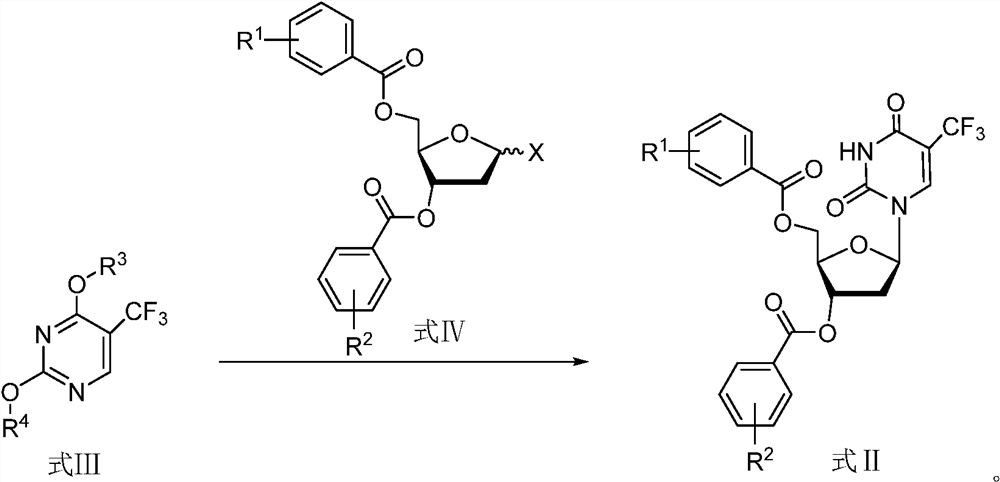
A kind of preparation method of trifluridine
A compound and selected technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of viscosity of the reaction system, difficult to stir, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The preparation of embodiment 1 formula III-1 compound
[0079]
[0080] Under the protection of nitrogen, add pre-dried acetonitrile (42kg), 5-trifluoromethyluracil (18kg), HMDS (19.8kg) and ammonium sulfate (796g) into a 200L reactor, and control the temperature of the feed liquid at 75°C to 85°C. ℃, stirring the reaction for 4h, and stopping the reaction. The feed solution is first controlled at 45-55°C and concentrated under reduced pressure to remove acetonitrile, and then heated to 65-75°C to continue to concentrate under reduced pressure to remove excess HMDS. After concentrating, 13.1 kg of anisole was added to the residue for dissolving, filtered, and the filtrate was collected to obtain 44.3 kg of anisole solution containing the compound of formula III-1 (wherein, the quality of the compound of formula III-1 was 31.2 kg, and the amount of benzyl The mass of ether is 13.1 kg).
Embodiment 2
[0081] The preparation of embodiment 2 formula II-1 compound
[0082]
[0083] Under the protection of nitrogen, add acetic anhydride (1.96kg) to the anisole solution containing the compound of formula III-1 prepared in Example 1, heat the system to 40°C-50°C, and add the formula IV-1 in 5 batches under stirring. 1 compound (9.5kg*5, α / β is about 60:40), add a batch every 0.5h, after the compound of formula IV-1 is added, control the temperature at 40°C-50°C and continue to stir for 2-3h, TLC monitors that the compound of formula III-1 has reacted completely, and the reaction is stopped, and the reaction system is syrupy and easy to stir.
[0084] Add 74 kg of absolute ethanol to the reaction system, stir to disperse the solid evenly, crystallize the system at -5-5°C for 7h-10h, and dry the filter cake at 45°C-55°C for 14-18h to obtain the crude compound of formula II-1 46.72 kg. (α:β=1:14.1)
[0085] Pump 654kg of absolute ethanol into a 1000L glass-lined reactor, add 4...
Embodiment 3
[0086] Example 3 Preparation of Trifluridine
[0087]
[0088] Under the protection of nitrogen, add 160kg of anhydrous methanol and 23.2kg of the refined compound of formula II-1 into a 300L reaction kettle, stir, cool down with chilled water, control the temperature of the system at -20°C to -10°C, and add the pre-prepared sodium methoxide dropwise Methanol (5.57kg sodium methoxide, 26kg methanol) solution, dropwise, continue to control the temperature and stir for 2h, after the reaction of the refined product of the compound of formula II-1 is monitored by TLC, control the temperature at -20°C to -10°C, and add acetic acid dropwise to adjust System pH to neutral.
[0089] Concentrate the reaction solution thus obtained to dryness under reduced pressure at 35°C-45°C, add 150 kg of acetone to the concentrate with stirring, stir for 1 hour, filter, and concentrate the filtrate to dryness at 30°C-40°C. The residue was beaten twice with 50 kg of ethyl acetate, filtered, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com