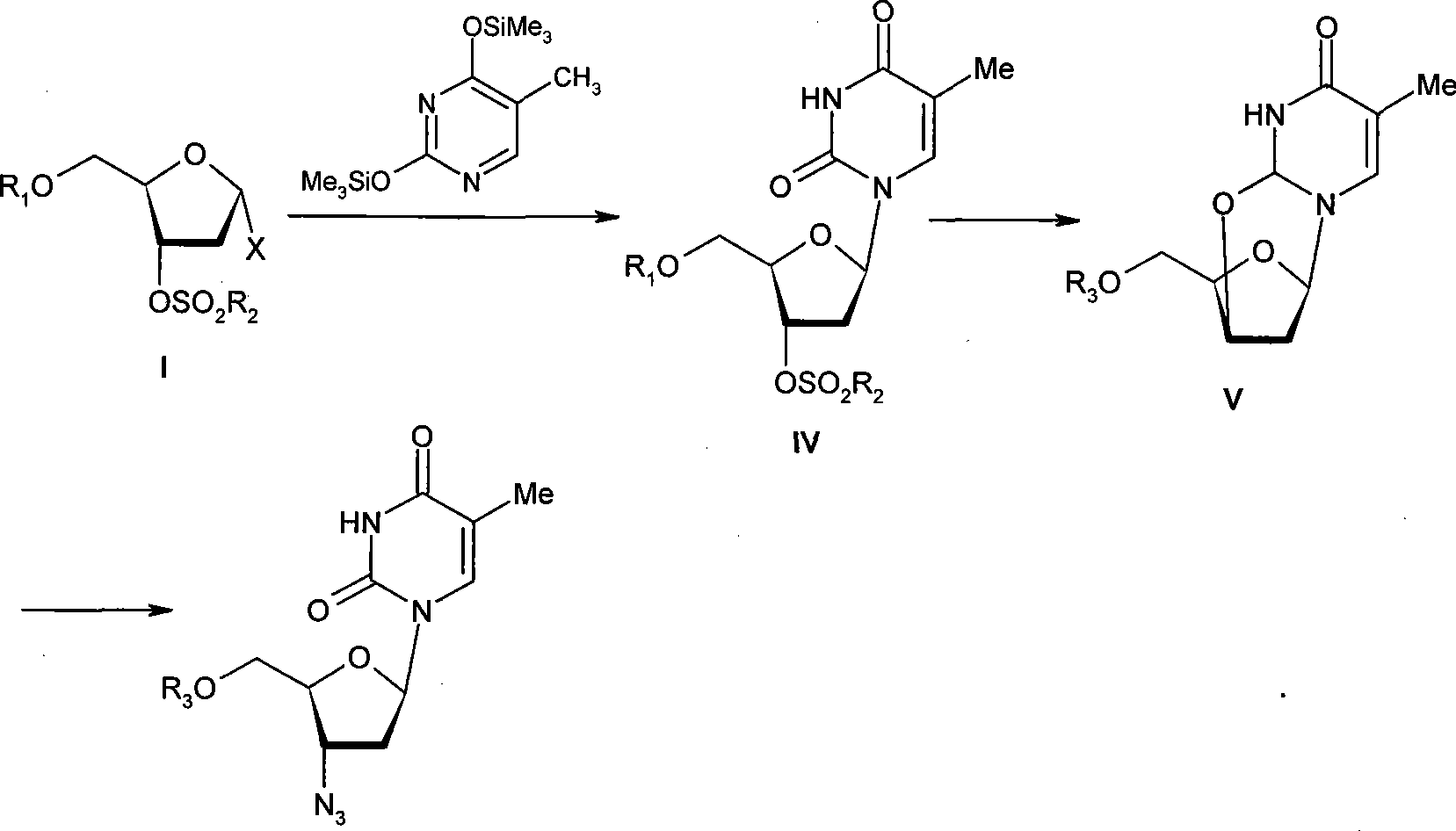
Intermediate for synthesizing azidothimidine, preparation thereof and use in azidothimidine synthesis
A kind of technology of zidovudine and intermediate, which is applied to the intermediate of synthesizing zidovudine and the preparation thereof and the application field of the intermediate in synthesizing zidovudine, can solve the problems of repetition, lengthy steps and the like, Achieve the effect of short route, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
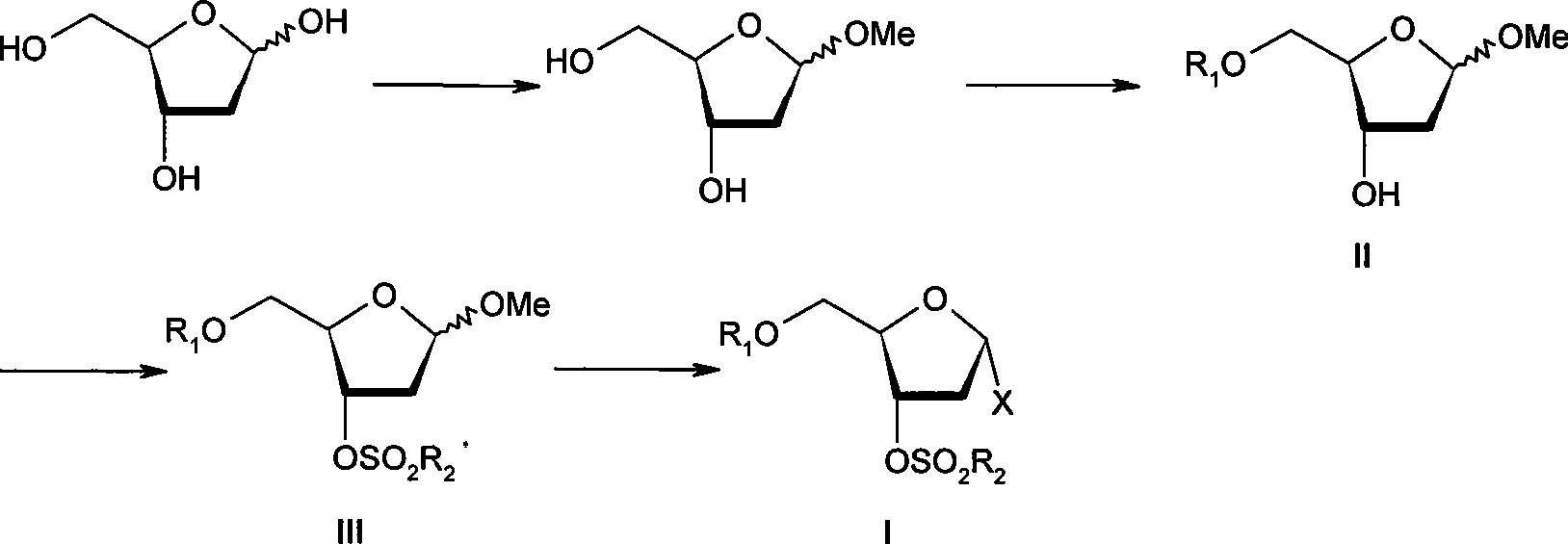
[0045] Methyl 2-deoxy-D-ribofuranoside
[0046] At room temperature, 2-deoxy-D-ribose (26.8 g, 0.20 mol) was dissolved in anhydrous methanol (300 ml), and stirred to dissolve. Add 1% hydrogen chloride-methanol solution in one portion. The stirring reaction was continued at room temperature for 3 hours, and the end point of the reaction was detected by TLC (dichloromethane:methanol=10:1). After the reaction was completed, sodium carbonate (24.5 g, 0.23 mol) was added to the reaction solution, and stirring was continued for 1 hour at room temperature. Methanol was distilled off under reduced pressure, dichloromethane (300ml) was added to the residue to dissolve, filtered, and insoluble matter was filtered off. The filtrate was concentrated to dryness to obtain 30.6 g of a light yellow oil.
Embodiment 2
[0048] Methyl 5-O-p-chlorobenzoyl-2-deoxy-D-ribofuranoside
[0049] At room temperature, the compound of Example 1 (23.4 g, 0.16 mol) was dissolved in dichloromethane (500 ml), and stirred evenly. The temperature was cooled to 0°C in an ice bath, and triethylamine (30.8ml, 0.22mol) was added. A solution of p-chlorobenzoyl chloride (24.4ml, 0.19mol) in dichloromethane (80ml) was slowly added dropwise under a controlled temperature of 0-5°C. The dropwise addition was completed in 4 hours, and the end point of the reaction was detected by TLC (ethyl acetate:petroleum ether=1:2). After the reaction was completed, the reaction solution was continuously stirred at 0-5° C. for 1 hour, and was directly used for the next reaction without any treatment. It can also be treated as follows: ice water (200ml) is added to the reaction solution and stirring is continued for 1 hour. Separate the organic phase, and wash the organic phase with saturated sodium bicarbonate solution (300ml×3) a...
Embodiment 3
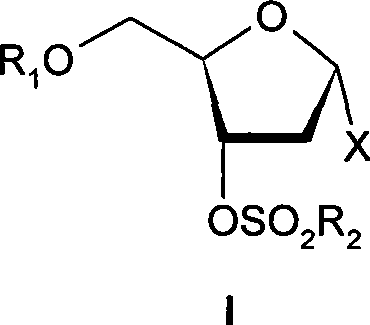
[0051] Methyl 3-O-methylsulfonyl-5-O-p-chlorobenzoyl-2-deoxy-D-ribofuranoside
[0052] At 0-5°C, triethylamine (30.8ml) was added to the reaction solution in Example 2, and stirred for 5 minutes. Methanesulfonyl chloride (21.7 g, 0.19 mol) in dichloromethane (50 ml) was added dropwise at a controlled temperature of 0-5°C. The dripping was completed in about 30 minutes, and the stirring was continued for 1 hour after the dripping, and the end point of the reaction was detected by TLC (ethyl acetate:petroleum ether=1:1). After the reaction was completed, ice water (200 ml) was added to the reaction solution, and stirring was continued for 1 hour. The organic phase was separated, and the organic phase was washed successively with 5% hydrochloric acid (200ml×3), saturated sodium bicarbonate solution (300ml×3), and saturated brine (300ml). Dry over anhydrous sodium sulfate. Filter and concentrate the filtrate to dryness under reduced pressure. Column chromatography gave 49.0 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com