Process for purifying zidovudine palmitate raw materials and preparations thereof
A technology of palmitate and zidovudine, applied in the field of medicine, can solve problems such as no manufacturer's production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
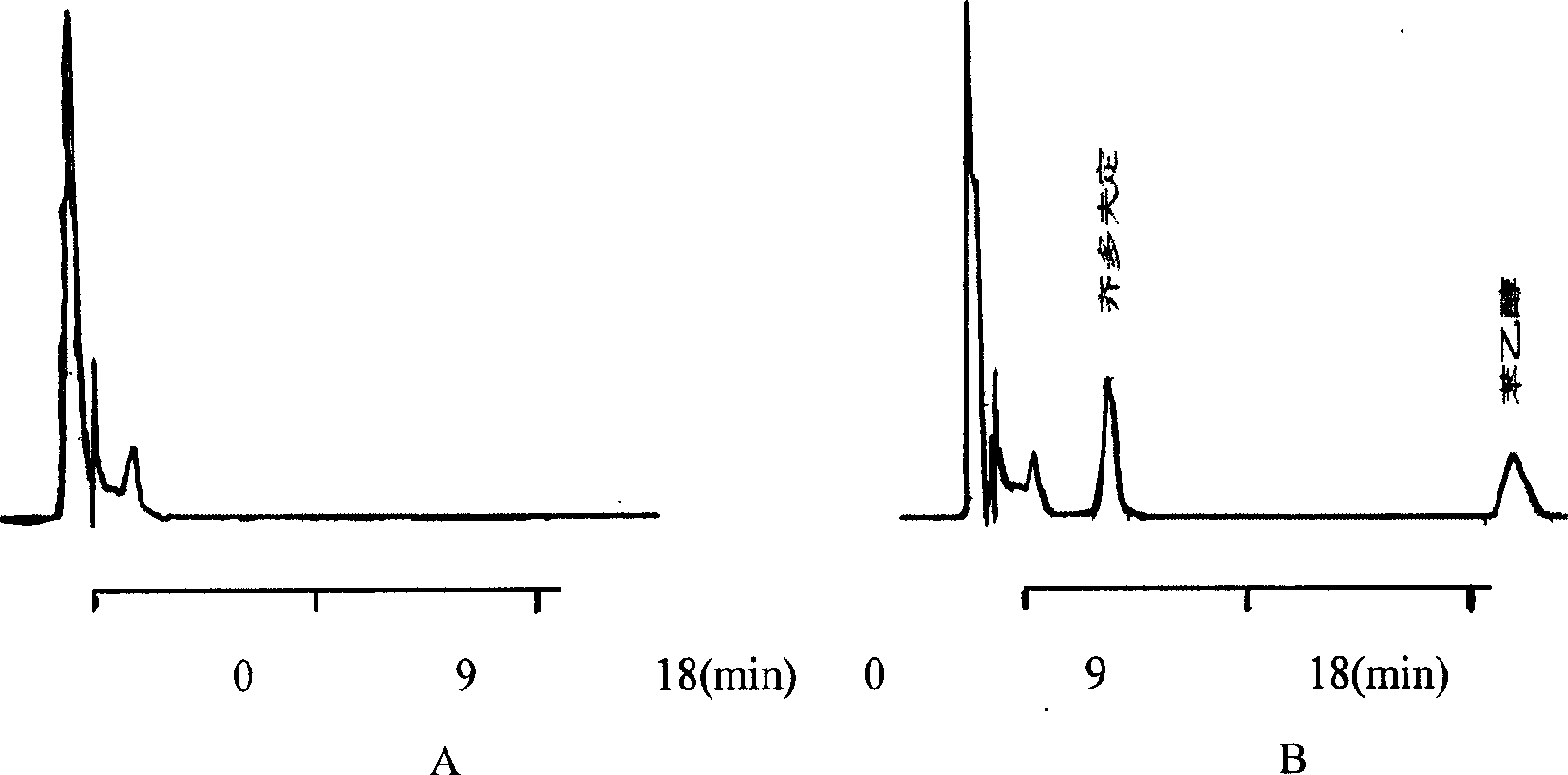
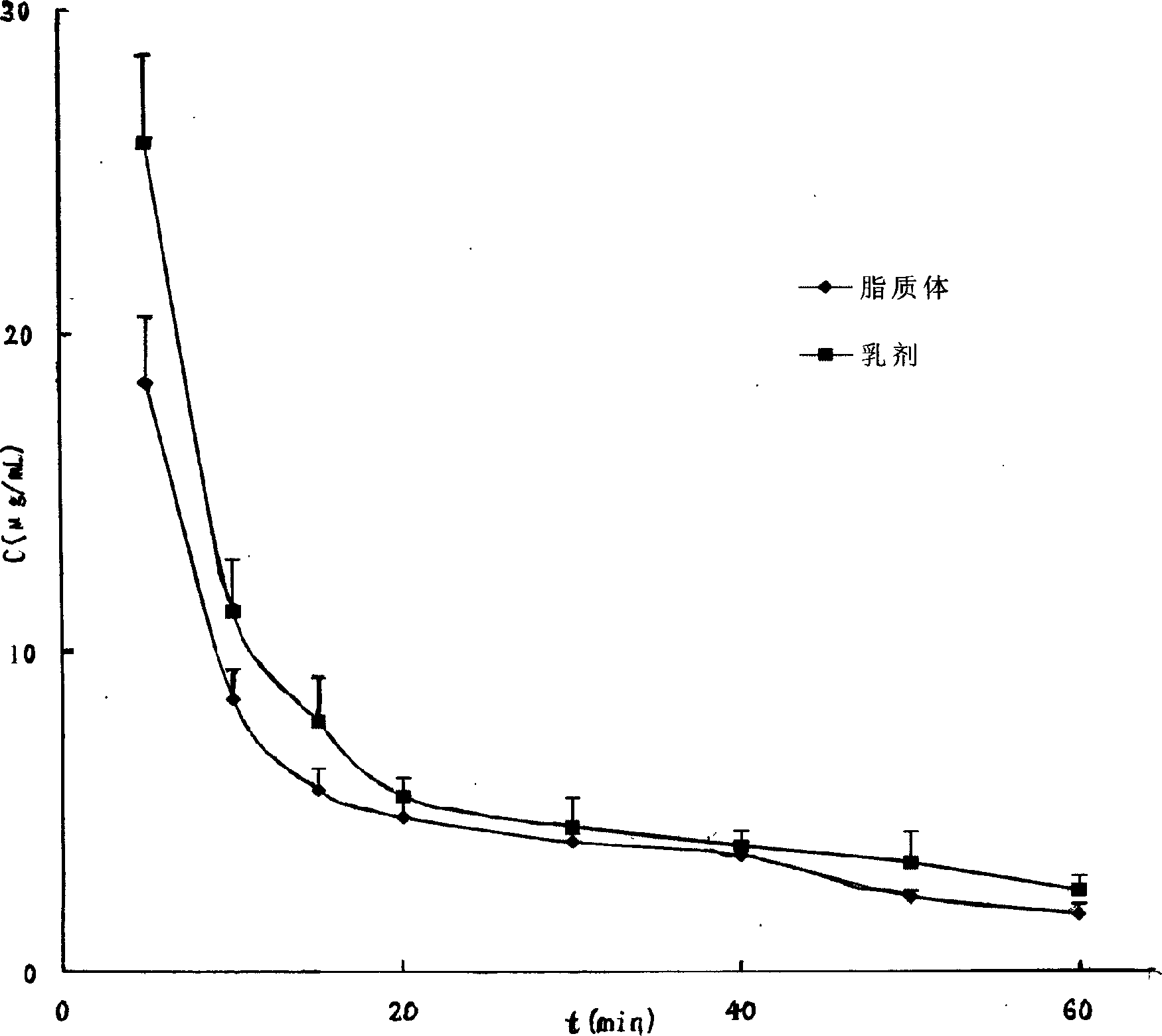
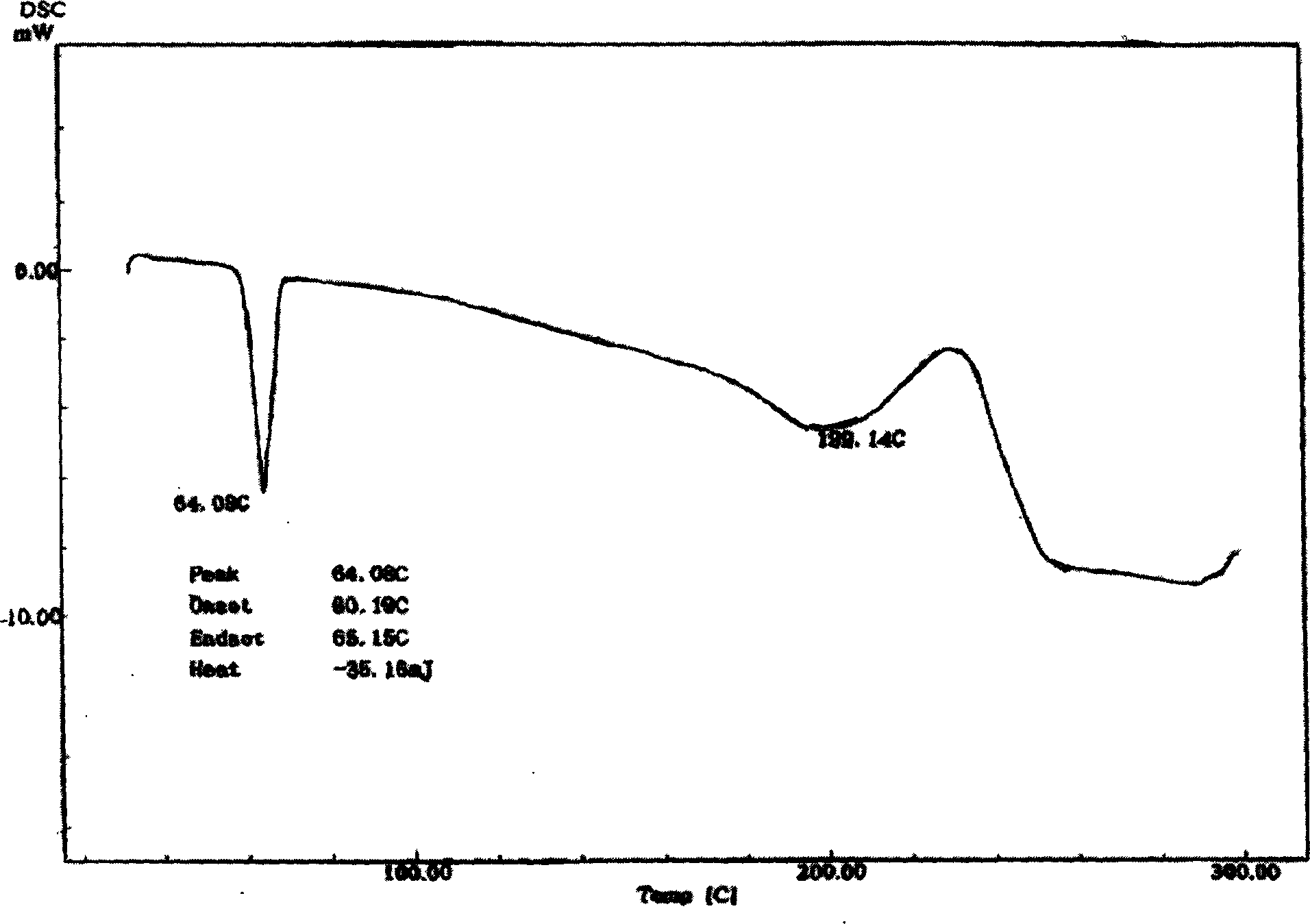
[0041] Take 10g of the synthetic zidovudine palmitate crude product, dissolve it in 400mL 95% ethanol at 50°C, add 0.5g of activated carbon, stir, mix well, remove the activated carbon by suction filtration, cool the filtrate, stir for 10 minutes, continue After cooling down to -20°C, a large amount of zidovudine palmitate precipitated after half an hour as needle crystals, and the pure product was obtained by suction filtration. The appearance is white, the related substance is 0.73% as determined by HPLC, and the purity is 99.27%.
Embodiment 2
[0043] Take 10g of the synthesized crude zidovudine palmitate, dissolve it in 300mL of methanol at 40°C, add 1g of silica gel, stir, mix well, remove the silica gel by suction filtration, cool the filtrate, stir for 10 minutes, and continue to cool down to - After 1 hour at 20°C, a large amount of zidovudine palmitate precipitated as needle crystals, and the pure product was obtained by suction filtration. The appearance is white, and the related substance is 0.78% as determined by HPLC, and the purity is 99.22%.
Embodiment 3
[0045] Take 10 g of the synthesized crude product of zidovudine palmitate, dissolve it in 100 mL of methanol-ethyl acetate (100:1) at 40 ° C, add 1 g of silica gel, stir, mix well, remove the silica gel by suction filtration, and cool the filtrate , stirred for 10 minutes, and continued to cool down to -20°C. After 1 hour, a large amount of zidovudine palmitate was precipitated as needle crystals, and the pure product was obtained by suction filtration. The appearance is white, and the related substance is 0.88% as determined by HPLC, and the purity is 99.12%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com