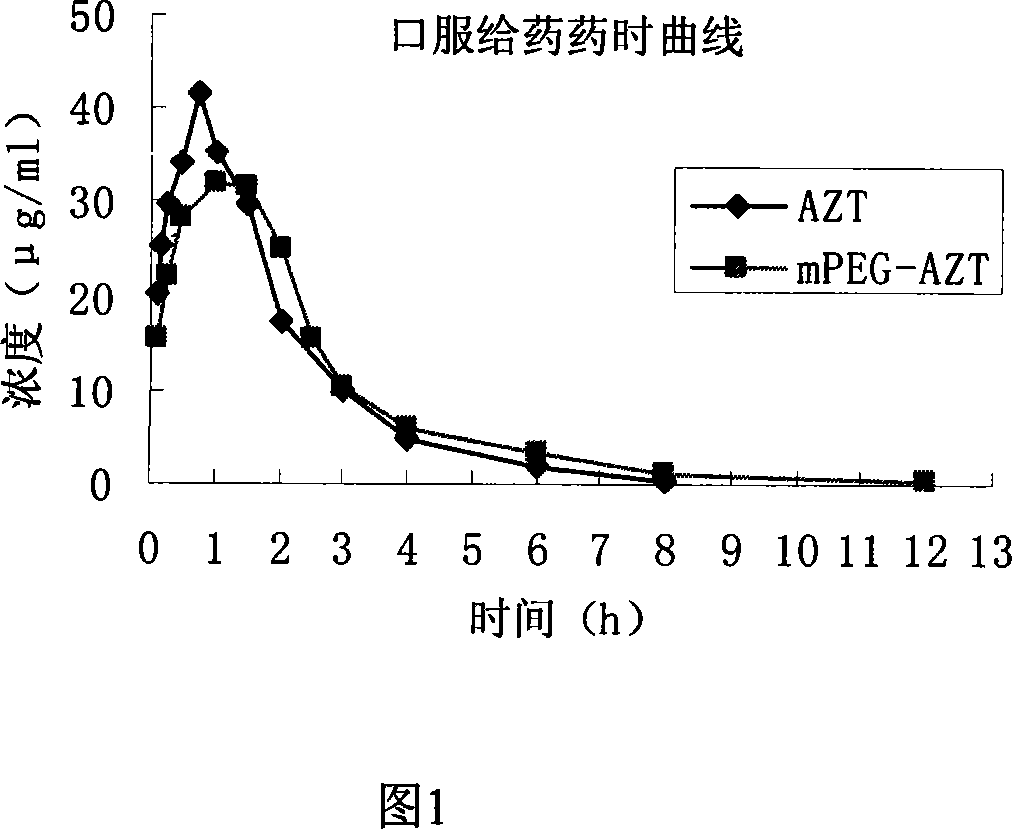
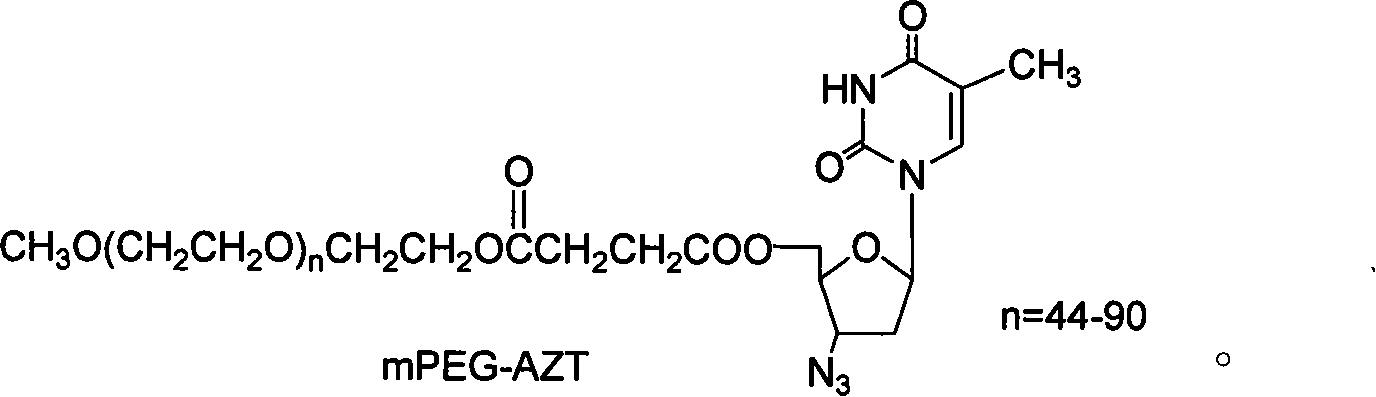
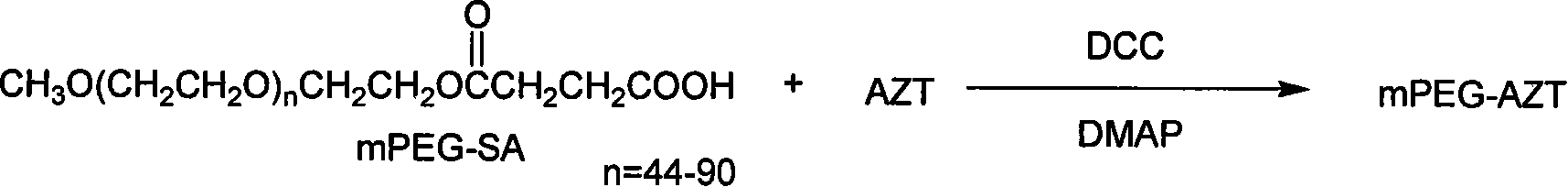Polyesthylene glycol modified zidovudine conjugate and its prepn process and application
A technology of polyethylene glycol and zidovudine, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of monomethoxy polyethylene glycol 2000 zidovudine conjugated prodrug
[0035] Put 1g mPEG2000 (0.5mmol) in a 50ml round-bottom flask, add 20ml of toluene, install the upper separator and steam out 10ml of toluene, then add 0.5g (5mmol) succinic anhydride to the round-bottom flask, continue heating and reflux for 5h , TLC detection (I 2 dyeing), after the reaction is completed, toluene is evaporated under reduced pressure, the solid is dissolved with 5 mL of dichloromethane and filtered, and 50 times the volume of glacial ether is added to the filtrate to obtain a white precipitate, which is monomethoxypolyethylene glycol succinic acid Monoester (mPEG-SA), 1.02 g was obtained, and the yield was 97.1%.
[0036] Add 0.134g (0.5mmol) of AZT, 0.015g (0.125mmol) of DMAP and 0.233g (1mmol) of DCC into a 50ml round bottom flask, add 5ml of dichloromethane and stir until dissolved, and dropwise add 5ml of 1.05g (0.5 mmol) mPEG-SA dichloromethane solutio...
Embodiment 2
[0042] Example 2: Preparation of monomethoxy polyethylene glycol 6000 zidovudine conjugated prodrug
[0043] Put 3g of mPEG6000 (0.5mmol) in a 50ml round bottom flask, add 20ml of toluene, install the upper water separator and steam out 10ml of toluene, then add 0.5g (5mmol) of succinic anhydride to the round bottom flask, continue heating and reflux for 5h , TLC detection (I 2 dyeing), after the reaction is completed, toluene is evaporated under reduced pressure, the solid is dissolved with 5 mL of dichloromethane and filtered, and 55 times the volume of glacial ether is added to the filtrate to obtain a white precipitate, which is monomethoxypolyethylene glycol succinic acid Monoester (mPEG-SA). Obtained 3.01 g, yield 98.7%.
[0044] Add 0.134g (0.5mmol) of AZT, 0.015g (0.125mmol) of DMAP and 0.233g (1mmol) of DCC into a 50ml round bottom flask, add 5ml of dichloromethane and stir until dissolved, and dropwise add 5ml of 3.05g (0.5 mmol) mPEG-SA dichloromethane solution. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com