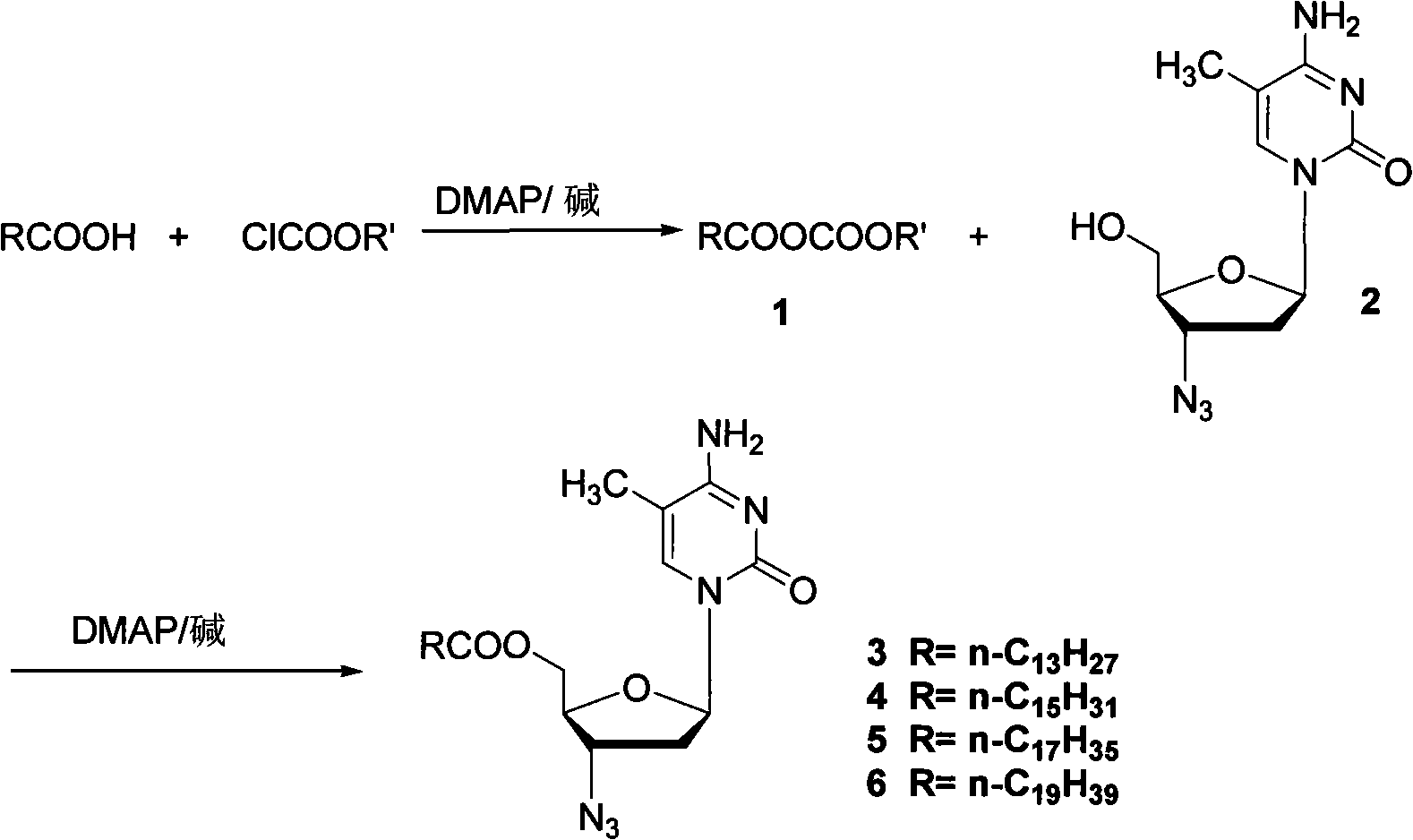
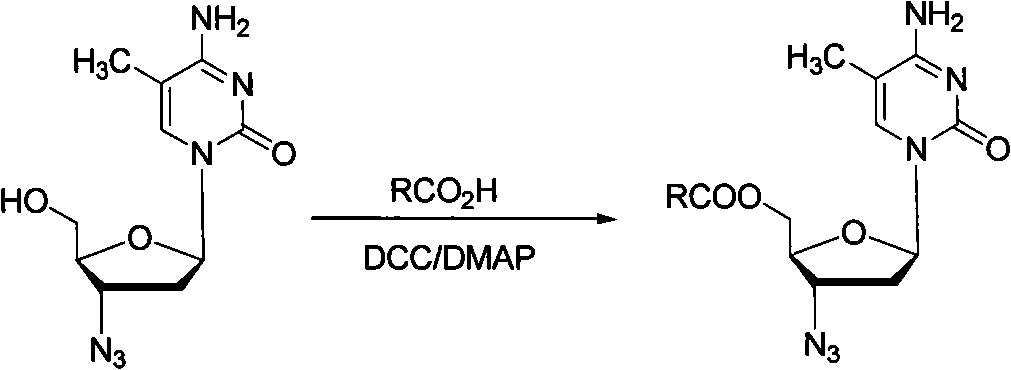
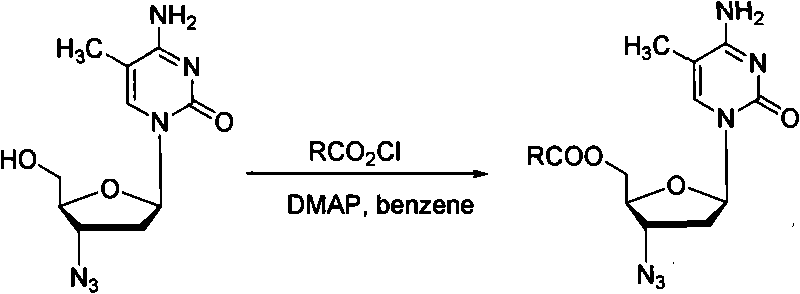
Method for synthesizing higher fatty acid zidovudine ester
A technology of higher fatty acid and zidovudine, which is applied in the direction of drug combination, sugar derivatives, organic chemistry, etc., can solve the problems of being unsuitable for industrial production, corrosive and toxic, difficult to separate and purify, etc., and achieve high yield , Improve stability, mild condition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step 1 Preparation of myristic acid mixed anhydride 1
[0026] Myristic acid (4.1 g, 18 mmol) and N,N-dimethyl-4-aminopyridine (440 mg, 3.6 mmol) were dissolved in dichloromethane (36 mL), triethylamine (3.7 mL, 27 mmol) was added, Ethyl chloroformate (1.8 mL, 19 mmol) was added dropwise at room temperature. The mixed solution was reacted at 40° C. for 12 h, cooled to room temperature and directly proceeded to the next reaction.
[0027] Step 2 synthesis of zidovudine myristate
[0028] Triethylamine (3.7mL, 27mmol) was added to the reaction solution obtained in step 1, and then zidovudine (4.8g, 18mmol) and N,N-dimethyl-4-aminopyridine (440mg, 3.6mmol) Dissolved in dichloromethane (9 mL) and added to the reaction solution through the dropping funnel. The mixed solution was reacted at 40° C. for 10 h and cooled to room temperature.
[0029] Step 3 Purification of zidovudine myristate
[0030] The reaction solution obtained in step 2 was filtered through diatomaceou...
Embodiment 2
[0032] Step 1 Preparation of palmitic acid mixed anhydride 1
[0033] Palmitic acid (9.2g, 36mmol) and N,N-dimethyl-4-aminopyridine (1.32g, 10.8mmol) were dissolved in tetrahydrofuran (120mL), triethylamine (7.4mL, 54mmol) was added, and Ethyl chloroformate (7.2 mL, 72 mmol) was added dropwise. The mixed solution was reacted at 68°C for 10 h, cooled to room temperature and directly proceeded to the next reaction.
[0034] Step 2 Synthesis of Zidovudine Palmitate
[0035] Triethylamine (7.4mL, 54mmol) was added to the reaction solution obtained in step 1, and then zidovudine (9.6g, 36mmol) and N,N-dimethyl-4-aminopyridine (1.32g, 10.8mmol ) was dissolved in tetrahydrofuran (18 mL) and added to the reaction solution through a dropping funnel. The mixed solution was reacted at 68°C for 7h and cooled to room temperature.
[0036] Purification of step 3 zidovudine palmitate
[0037] The reaction solution obtained in step 2 was filtered through diatomaceous earth to remove insolu...
Embodiment 3
[0039] Step 1 prepares stearic acid mixed anhydride 1
[0040] Dissolve stearic acid (5.1g, 18mmol) and N,N-dimethyl-4-aminopyridine (0.11g, 9mmol) in 1,2 dichloroethane (18mL), add pyridine (3.6mL, 45mmol ), ethyl chloroformate (8.5 mL, 90 mmol) was added dropwise at room temperature. The mixed solution was reacted at 84°C for 8h, cooled to room temperature and directly proceeded to the next reaction.
[0041] Step 2 synthesizes zidovudine stearate
[0042] Pyridine (3.6mL, 45mmol) was added to the reaction solution obtained in step 1, then zidovudine (4.8g, 18mmol) and N,N-dimethyl-4-aminopyridine (0.11g, 9mmol) were dissolved in 1,2 Dichloroethane (18 mL) was added to the reaction solution through the dropping funnel. The mixed solution was reacted at 84°C for 5h and cooled to room temperature.
[0043] The purification of step 3 zidovudine stearate
[0044] The reaction solution obtained in step 2 was filtered through diatomaceous earth to remove insoluble solids, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com