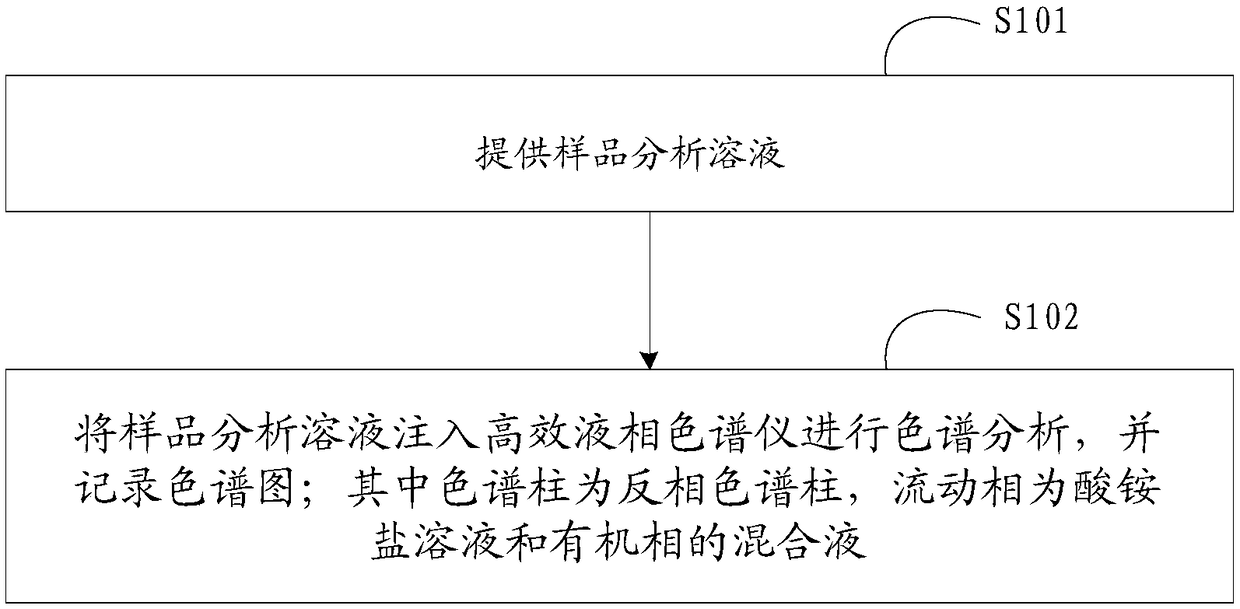
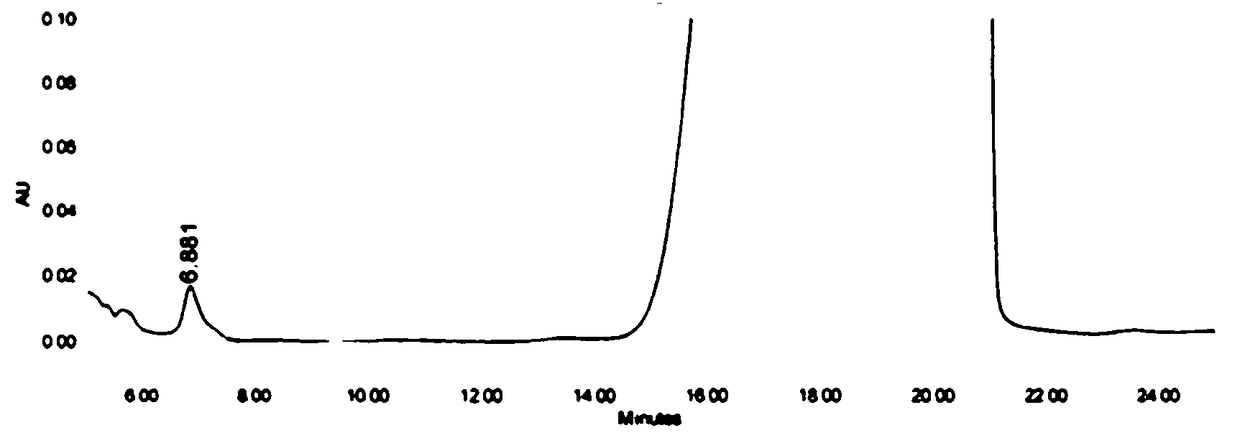
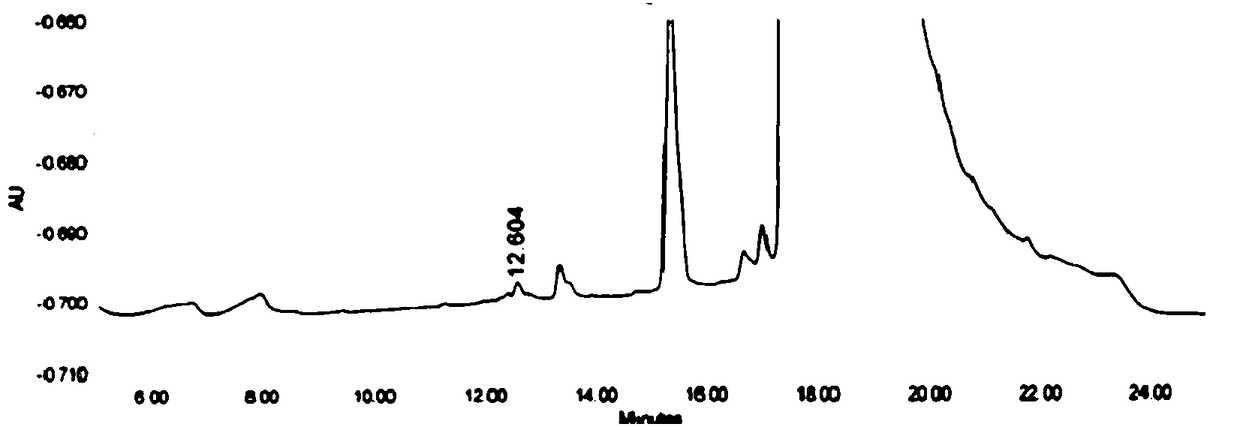
High-performance liquid detection method for genotoxic impurities of zidovudine
A technology of zidovudine and genotoxicity, which is applied in the field of drug analysis, can solve the problems of inability to quantitatively control genotoxic impurities, low limits of genotoxic impurities, and the influence of quantitative tailing, etc., to improve the detection resolution and detection limit, The effect of increasing the service life and reducing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0050] (1) Instrument and chromatographic conditions
[0051] High performance liquid chromatography: e2965 high performance liquid chromatography system and workstation.
[0052] Chromatographic column: Thermo Syncronis C18 (4.6mm×250mm, 5μm) octadecylsilane bonded silica gel chromatographic column.
[0053] Mobile phase: prepare 0.020mol / L ammonium acetate solution, adjust the pH value to 4.5 with glacial acetic acid, and mix the ammonium acetate solution with methanol as the water phase to obtain the mobile phase; wherein, the ratio of water phase-methanol in the mobile phase is as follows: At time points of 0, 8, 20, 25, and 35 minutes, the volume ratio of the aqueous phase was 90%, 35%, 10%, 90%, and 90% for mixing.
[0054] Detection conditions: set the flow rate of the mobile phase to 1.0ml / min, the detection wavelength to 265nm, and the column temperature to 35°C.
[0055] (2) Experimental steps
[0056] Prepare sample analysis solution:
[0057] Take predetermined...
experiment example 2
[0062] (1) Instrument and chromatographic conditions
[0063] High performance liquid chromatography: e2965 high performance liquid chromatography system and workstation.
[0064] Chromatographic column: Thermo Syncronis C18 (4.6mm×250mm, 5μm) octadecylsilane bonded silica gel chromatographic column.
[0065] Mobile phase: prepare 0.020mol / L ammonium acetate solution, adjust the pH value to 5.0 with glacial acetic acid, and mix the ammonium acetate solution with methanol as the water phase to obtain the mobile phase; wherein, the ratio of water phase-methanol in the mobile phase is as follows: At time points of 0, 8, 20, 25, and 35 minutes, the volume ratio of the aqueous phase was 90%, 30%, 5%, 90%, and 90% for mixing.
[0066] Detection conditions: set the flow rate of the mobile phase to 1.0ml / min, the detection wavelength to 265nm, and the column temperature to 40°C.
[0067] (2) Experimental steps
[0068] Prepare sample analysis solution:
[0069] Take predetermined ...
experiment example 3
[0074] (1) Instrument and chromatographic conditions
[0075] High performance liquid chromatography: e2965 high performance liquid chromatography system and workstation.
[0076] Chromatographic column: Agilent ZORBAX SB-CN (4.6mm×250mm, 5μm) cyano-bonded silica gel chromatographic column.
[0077] Mobile phase: prepare 0.020mol / L ammonium acetate solution, adjust the pH value to 5.5 with glacial acetic acid, and mix the ammonium acetate solution with methanol as the water phase to obtain the mobile phase; wherein, the ratio of water phase-methanol in the mobile phase is as follows: At time points of 0, 8, 20, 25, and 35 minutes, the volume ratio of the aqueous phase was 90%, 30%, 5%, 90%, and 90% for mixing.
[0078] Detection conditions: set the flow rate of the mobile phase to 1.0ml / min, the detection wavelength to 265nm, and the column temperature to 40°C.
[0079] (2) Experimental steps
[0080] Prepare sample analysis solution:
[0081] Take predetermined amounts of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com