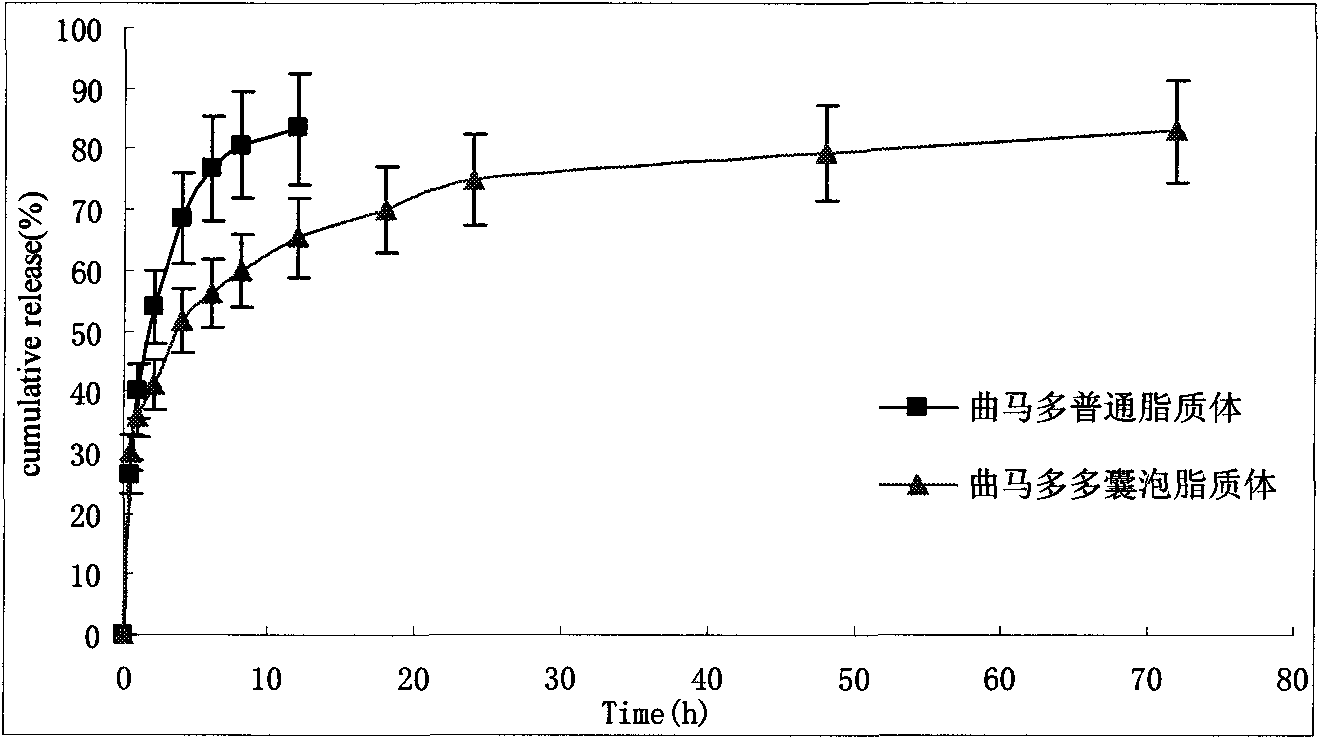
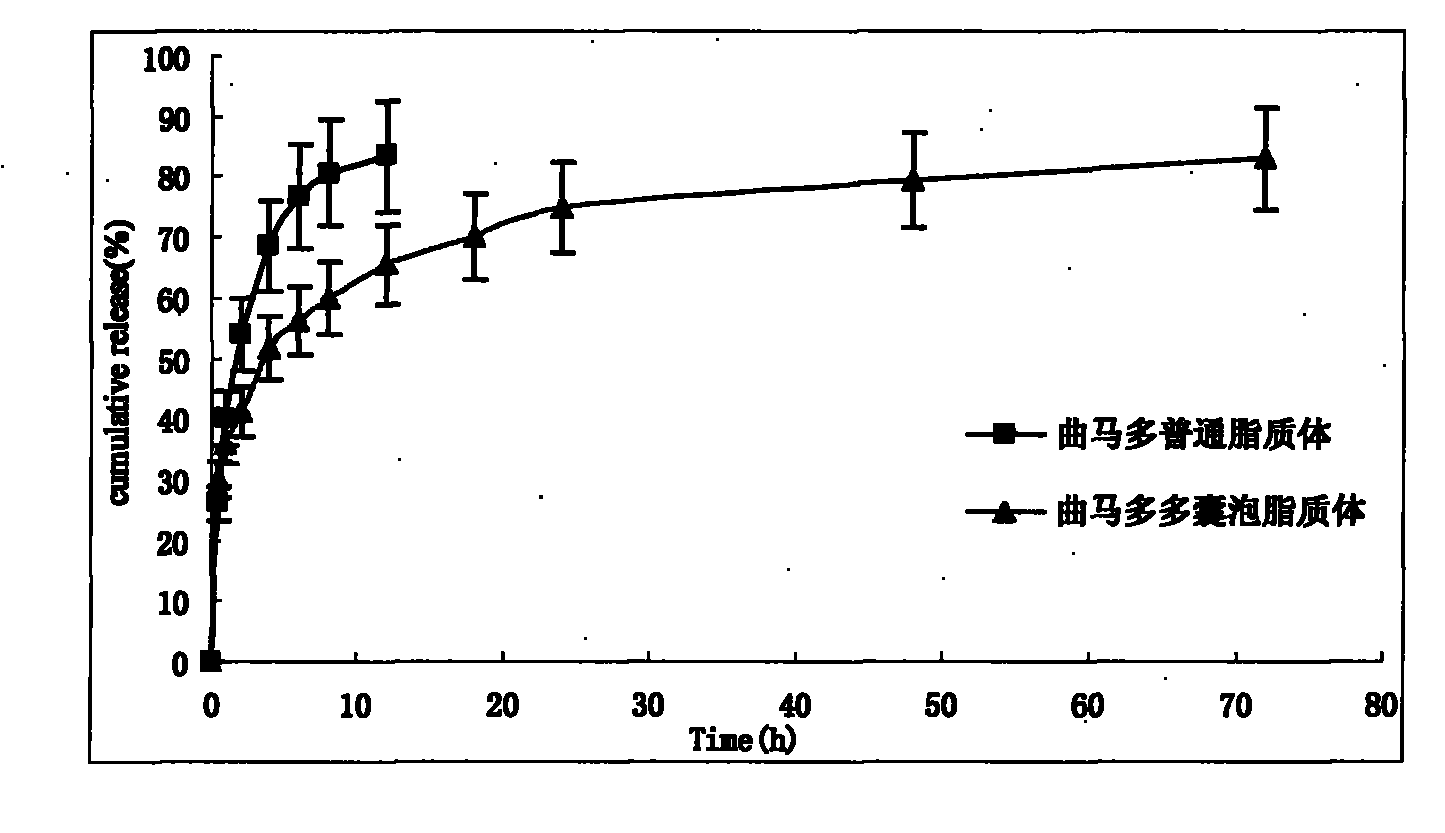
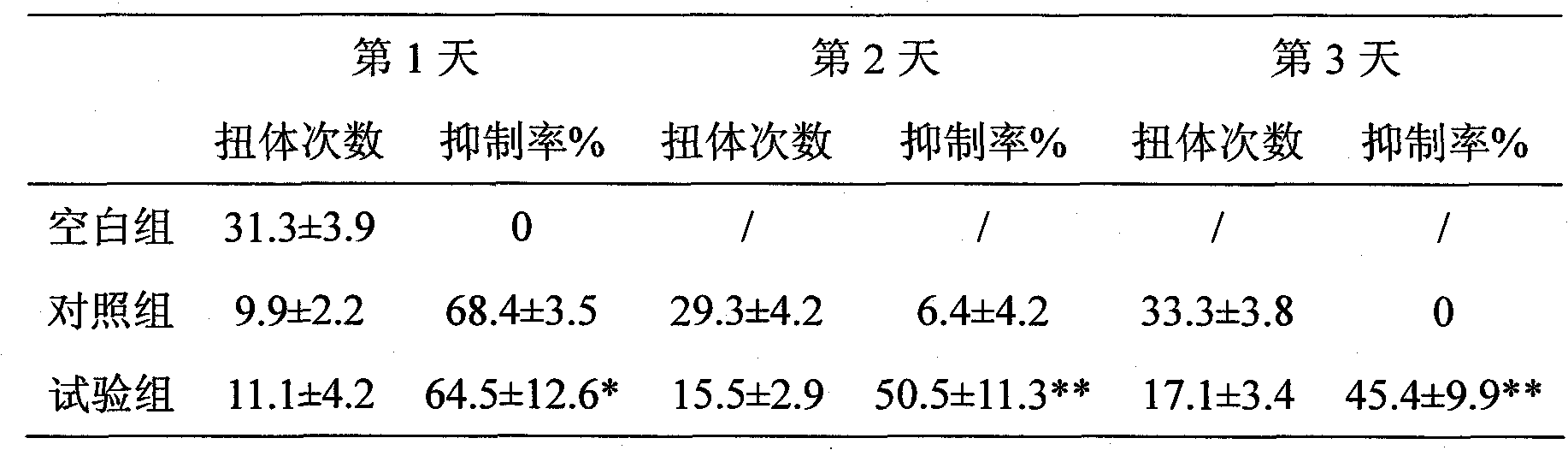
Tramadol multivesicular liposome and preparation method thereof
A technology of tramadol and liposome, which is applied in the field of tramadol vesicle liposome and its preparation, can solve the problems of preparation encapsulation efficiency and drug release difference, and achieves reduction of total dosage, reduction of medication times, Good sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Weigh 50 μmol of soybean lecithin, 80 μmol of dioleoylphosphatidylcholine (DOPC), 50 μmol of dipalmitoylphosphatidylglycerol (DPPG), 100 μmol of cholesterol (CH), and 12 μmol of triolein (To) in proportion. In the volumetric flask, add 5ml of chloroform to make it dissolve completely and become the organic phase;
[0029] 2. Prepare 5ml of tramadol hydrochloride solution with a concentration of 500mmol / L as the inner aqueous phase;
[0030] 3. Take 5ml of the inner aqueous phase and slowly drop it into 5ml of the organic phase in step 1, mix and emulsify, and ultrasonicate for 5 minutes to form water-in-oil (W / O) colostrum, which is observed as a milky white translucent viscous liquid;
[0031] 4. Prepare a solution containing 3% glucose, 0.2% Span 20, and arginine with a molar concentration of 40mmol / L as the external water phase, and inject 25mL of the external water phase into the prepared W / O colostrum, Emulsified and ultrasonicated for 5 minutes to obtain a wat...
Embodiment 2
[0036] 1. Weigh 80 μmol of hydrogenated soybean lecithin, 50 μmol of dioleoylphosphatidylcholine (DOPC), 200 μmol of dipalmitoylphosphatidylglycerol (DPPG), 120 μmol of cholesterol (CH), and 10 μmol of triolein (To), Place in a volumetric flask, add ether: chloroform (volume ratio 1:1) 5ml to make it completely dissolved, and become the organic phase;
[0037] 2. Prepare 5ml of tramadol hydrochloride solution with a molar concentration of 10mmol / L to form the inner aqueous phase;
[0038] 3. Take 5ml of the above-mentioned internal water phase and slowly drop it into 5ml of the organic phase in step 1, mix and emulsify, and ultrasonicate for 10 minutes to form water-in-oil (W / O) colostrum, which is observed as a milky white translucent viscous liquid;
[0039] 4. Prepare the solution containing 0.9% sodium chloride, 0.01% Tween 80, and the arginine with a molar concentration of 50mmol / L as the external water phase, and inject the external water phase into the colostrum of the ...
Embodiment 3
[0044] 1. Weigh 30 μmol of lecithin, 80 μmol of dioleoylphosphatidylethanolamine, 50 μmol of dipalmitoylphosphatidylglycerol, 10 μmol of phosphatidylglycerol, 30 μmol of cholesterol, 12 μmol of triolein, 10 μmol of phosphatidylserine, and 10 μmol of phosphatidic acid, Put it in a volumetric flask, add 5ml of chloroform to dissolve it completely, and become the organic phase.
[0045] 2. Prepare 5ml of tramadol hydrochloride solution with a concentration of 500mmol / L as the inner water phase.
[0046] 3. Take 5ml of the inner aqueous phase and slowly drop it into 5ml of the organic phase in step 1, mix and emulsify, and ultrasonicate for 5 minutes to form water-in-oil (W / O) colostrum, which is observed as a milky white translucent viscous liquid;
[0047] 4. Prepare 3% mannitol, 0.1% polyvinyl alcohol, L-lysine solution with a molar concentration of 20mmol / L as the external water phase, and inject 25mL of external water into the prepared W / O colostrum phase, emulsified and ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com