Preparation method of insoluble drug nanosuspension
A technology of insoluble drugs and nano-suspensions, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of complex preparation process and low drug concentration, and achieve the The effect of simple process, good reproducibility and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 paliperidone palmitate nanosuspension:
[0036] (1) Disperse 2.4g of Tween-20 and 1.5g of PEG4000 in 100mL of water for injection to obtain a dispersion medium containing a stabilizer.
[0037] (2) Disperse 23.4 g of paliperidone palmitate in the dispersion medium containing a stabilizer in step (1) to obtain an initial suspension with a drug concentration of 234 mg / mL.
[0038] (3) The primary suspension obtained in step (2) was sheared at a high speed of 22000 rpm for 20 minutes in an ice-water bath to obtain a thick suspension of paliperidone palmitate.
[0039] (4) The suspension obtained in step (3) was subjected to micro-jet high-pressure homogenization, the homogenization pressure was 30,000 psi, and the number of cycles was 20 times to obtain the final product paliperidone palmitate nanosuspension.
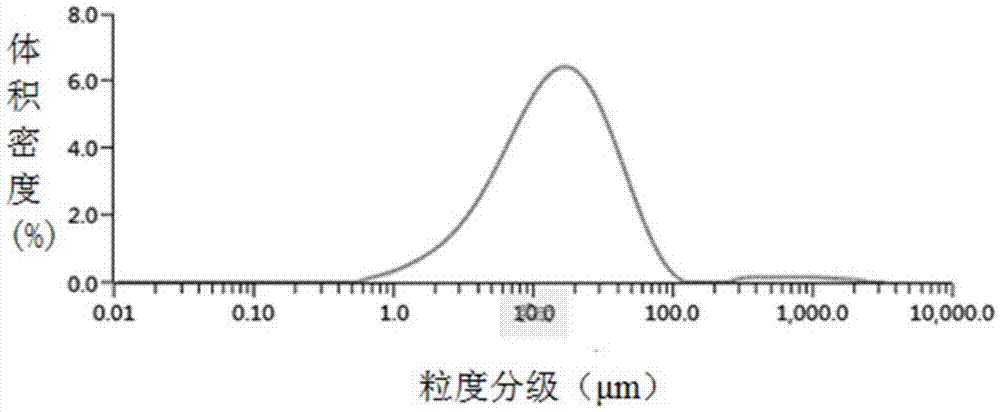
[0040] Using a Malvern Mastersizer 3000 laser particle size analyzer to determine paliperidone palmitate API powder D 50 14.8μm (p...
Embodiment 2
[0041] The preparation of embodiment 2 paliperidone palmitate nanosuspension:
[0042] (1) Disperse 2 g of lecithin and 8 g of polyvinylpyrrolidone-K30 (PVP-K30) in 100 mL of water for injection to obtain a dispersion medium containing a stabilizer.
[0043] (2) Disperse 25 g of paliperidone palmitate in the dispersion medium containing a stabilizer in step (1) to obtain an initial suspension with a drug concentration of 250 mg / mL.
[0044] (3) The primary suspension obtained in step (2) was sheared at a high speed of 22000 rpm for 20 minutes in an ice-water bath to obtain a thick suspension of paliperidone palmitate.
[0045] (4) The coarse suspension obtained in step (3) was subjected to high-pressure micro-jet homogenization, the homogenization pressure was 22000 psi, and the number of cycles was 30 times to obtain the final product paliperidone palmitate nanosuspension.
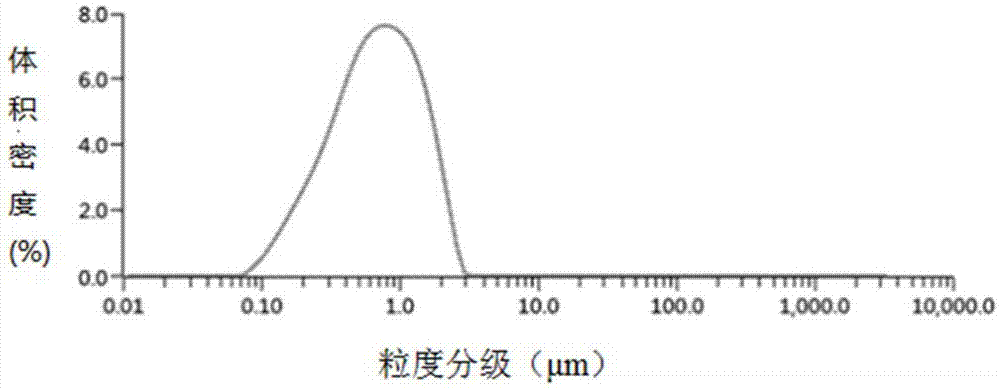
[0046] Using Malvern Mastersizer 3000 laser particle size analyzer to determine the drug particle siz...
Embodiment 3
[0047] The preparation of embodiment 3 curcumin dicaprate nanosuspensions:
[0048] (1) Disperse 3 g of Tween-80 and 0.75 g of sodium dodecylsulfonate in 100 mL of water for injection to obtain a dispersion medium containing a stabilizer.
[0049] (2) Disperse 30 g of curcumin dicaprate in the dispersion medium containing a stabilizer in step (1) to obtain an initial suspension with a drug concentration of 300 mg / mL.
[0050] (3) The primary suspension obtained in step (2) was sheared at 22000 rpm for 30 minutes in an ice-water bath to obtain D 50 Curcumin dicaprate thick suspension of 20μm.
[0051] (4) The coarse suspension obtained in step (3) was subjected to micro-jet high-pressure homogenization, the homogenization pressure was 35000 psi, and the number of cycles was 15 times to obtain the final product curcumin dicaprate nanosuspension.
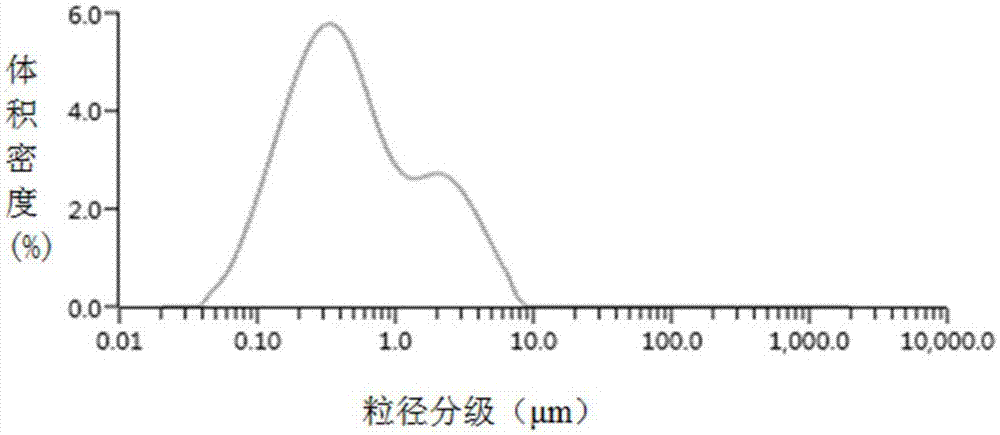
[0052] Use Malvern Mastersizer 3000 laser particle size analyzer to determine the drug particle size D in curcumin dicaprate nanosu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com