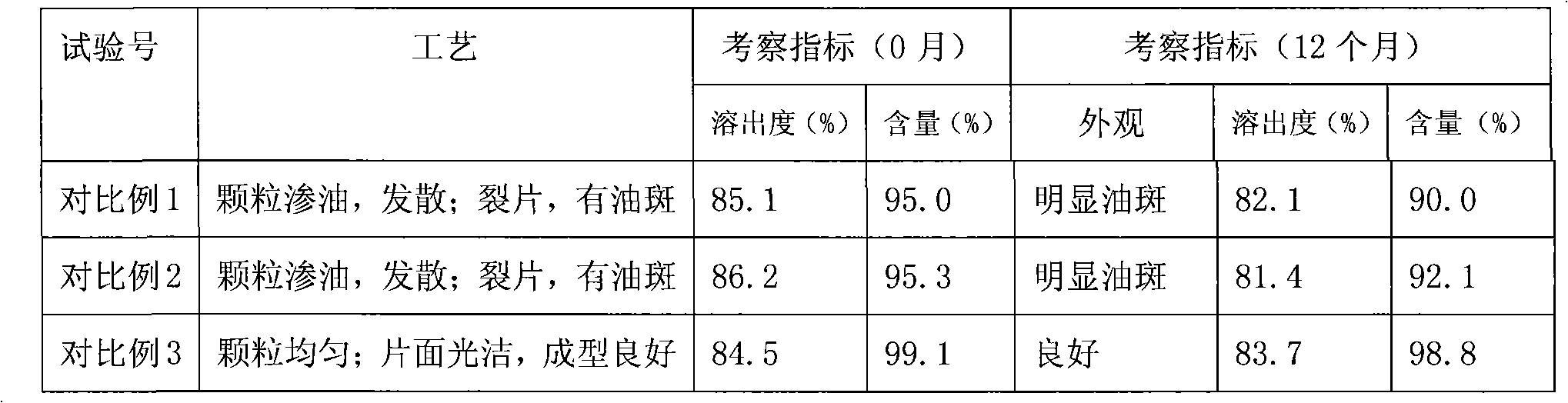
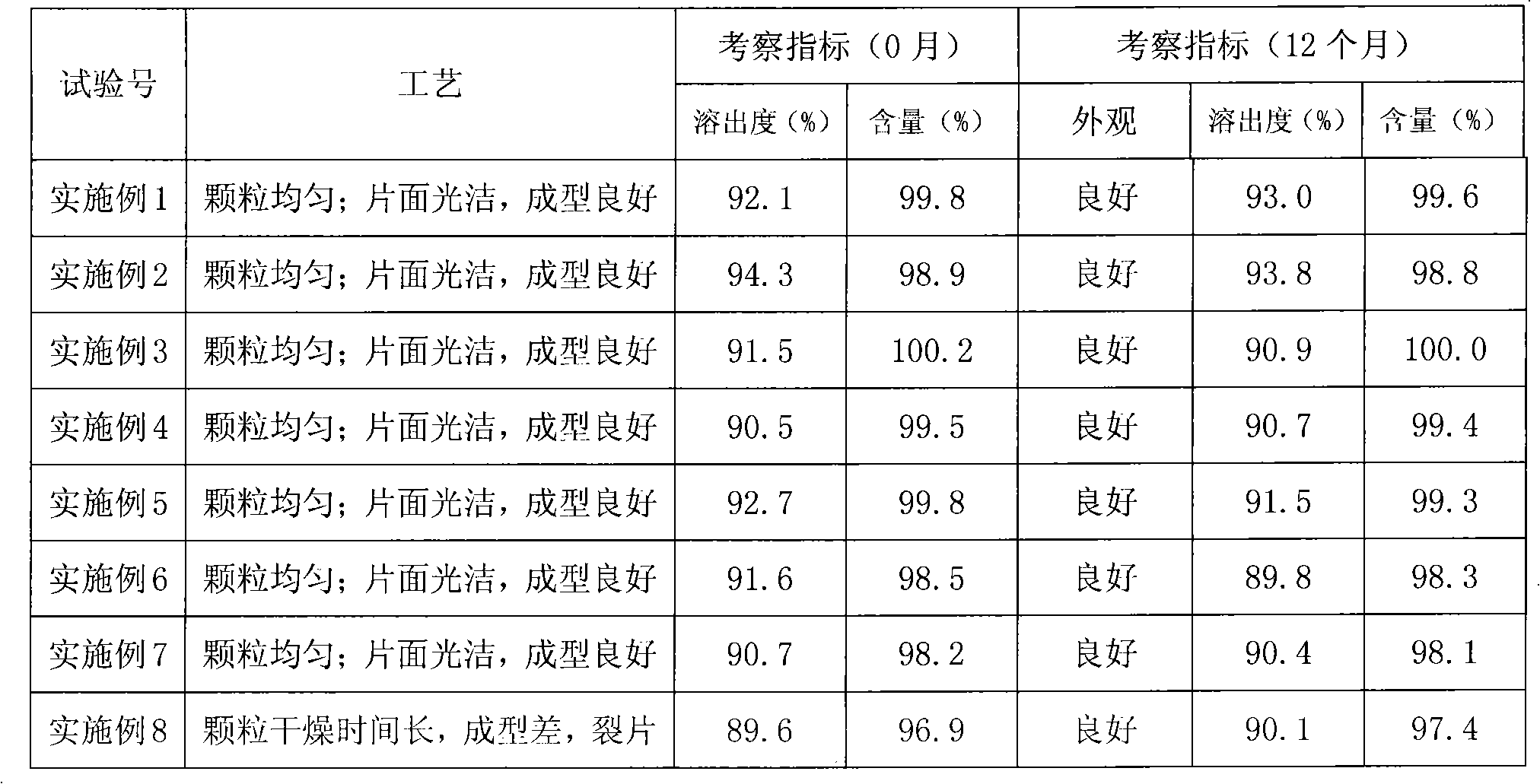
Butylbenzene phthalein tablet and preparation method thereof
A technology of butylphthalide tablets and butylphthalide, which is applied in the direction of medical formula, medical preparations containing active ingredients, pill delivery, etc., and can solve problems such as no formula given
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Prescription composition (5000 tablets)
[0053] Tablet core weight (g) weight percentage (%)
[0054] Butylphthalide 100.0 7.79
[0055]Poloxamer 0.1 0.01
[0056] β-cyclodextrin 899.9 70.03
[0057] Starch 100.0 7.78
[0058] Croscarmellose Sodium 150.0 11.67
[0059] Talc 23.0 1.79
[0060] Magnesium stearate 12.0 0.93
[0061] Aqueous solution of hydroxypropyl methylcellulose (2%) Appropriate amount
[0062] Total 1285.0 100
[0063] Preparation method: Weigh butylphthalide raw material and emulsifier poloxamer, add a small amount of water to stir and mix evenly, weigh β-cyclodextrin, add water to dissolve, mix the two solutions, stir rapidly for 1 hour, and refrigerate in the refrigerator ( 5°C) for 12 hours, take out and filter, and dry the obtained solid at 60°C to obtain solid powder of butylphthalide.
[0064] Weigh an appropriate amount of butylphthalide solid powder, add lactose and partly crosslinked carmellose sodium, mix well, granulate with hydro...
Embodiment 2
[0067] Prescription composition (5000 tablets)
[0068] Tablet core weight (g) weight percentage (%)
[0069] Butylphthalide 100.0 7.97
[0070] Poloxamer 1.0 0.08
[0071] β-cyclodextrin 899.0 71.63
[0072] Starch 120.0 9.56
[0073] Croscarmellose Sodium 100.0 7.97
[0074] Micropowder silica gel 23.0 1.83
[0075] Magnesium stearate 12.0 0.96
[0076] Appropriate amount of water
[0077] Total 1255.0 100
[0078] The preparation method is the same as in Example 1.
Embodiment 3
[0080] Prescription composition (5000 tablets)
[0081] Tablet core weight (g) weight percentage (%)
[0082] Butylphthalide 250.0 19.98
[0083] Polyethylene glycol-12-hydroxystearate 0.42 0.03
[0084] Hydroxypropyl-β-cyclodextrin 750.0 59.95
[0085] Lactose 165.6 13.24
[0086] Crospovidone 62.5 5.00
[0087] Talc 15.0 1.20
[0088] Magnesium stearate 7.5 0.60
[0089] Proper amount of povidone aqueous solution (5%)
[0090] Total 1251.02 100
[0091] Preparation method: Add butylphthalide and polyethylene glycol-12-hydroxystearate into water, stir and mix evenly; weigh hydroxypropyl-β-cyclodextrin and dissolve in water. The above two solutions are mixed, stirred rapidly, and the liquid medicine is dried by a spraying method to obtain a solid powder of butylphthalide.
[0092] Weigh an appropriate amount of butylphthalide solid powder, add pregelatinized starch and crospovidone, mix evenly, granulate with 5% povidone aqueous solution, dry, granulate, add magnesium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com