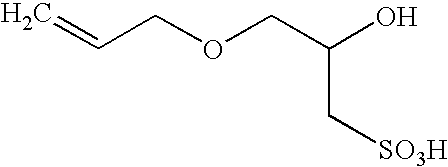
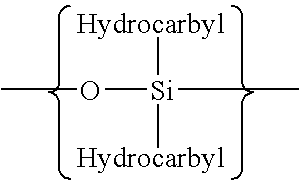
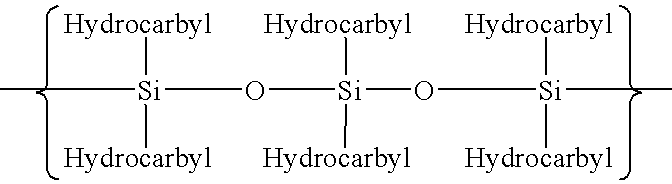Polymerization of fluoromonomers using a 3-allyloxy-2-hydroxy-1-propanesulfonic acid salt as surfactant
a technology of fluoromonomers and surfactants, applied in the field of polymerization of fluoromonomers, can solve the problems of affecting the molecular weight and other properties of products, the increasing scrutiny of perfluorinated surfactants, and the reduction of the reaction ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Vinylidene fluoride polymerization (AHPS salt surfactant).
Into a 7.5 liter, a horizontally disposed stainless steel reactor was charged 4.100 kg of deionized water, 0.004 kg of paraffin wax, 0.400 kg of an aqueous solution of AHPS sodium salt (0.500 wt. %), and 0.0500 kg of an aqueous solution containing the radical initiator potassium persulfate (2.00 wt. %) and the buffering agent sodium acetate (2.00 wt. %). The reactor was purged with argon and agitated for 30 minutes at room temperature. The reactor was then sealed and heated to about 80° C. VF2 monomer (0.438 kg) was charged into the reactor to a pressure of about 4,480 kPa. The reactor temperature stabilized at about 82° C. Additional amounts of the initiator / buffer solution were added after 4 minutes (0.0300 L), and after 14 minutes (0.0200 L). Time intervals recorded for the reaction were measured from the time that the reactor was fully pressurized with the monomer. The reactor pressure was maintained at 4480 kPa by the ad...
example 2
Vinylidene fluoride polymerization (AHPS salt surfactant).
Into a 7.5 liter, horizontally disposed stainless steel reactor were charged 4.100 kg of deionized water, 0.004 kg of paraffin wax, 0.400 kg of an aqueous solution of AHPS sodium salt (0.258 wt. %), and 0.080 kg of an aqueous solution containing the radical initiator potassium persulfate (2.00 wt. %) and the buffer sodium acetate (2.00 wt. %). The reactor was purged with argon and agitated for 30 minutes at room temperature. The reactor was then sealed and heated to about 80° C. VF2 monomer (0.443 kg) was charged into the reactor to a pressure of about 4,480 kPa. The reactor temperature stabilized at about 82° C. The reactor pressure was maintained at 4480 kPa by the addition of VF2 als needed. The feed of monomer was stopped at 2.0 hours, after 2.380 kg VF2 had been fed to the reactor. Reaction temperature and agitation were maintained for another 0.3 hour. The reactor was then cooled to room temperature and vented. The reac...
example 3
Vinylidene fluoride polymerization (AHPS salt surfactant).
Into a 7.5 liter, horizontally disposed stainless steel reactor were charged 4.740 kg of deionized water, 0.004 kg of paraffin wax, and 0.200 kg of an aqueous solution of AHPS sodium salt (3.00 wt. %). The reactor was purged with argon and agitated for 30 minutes at room temperature. The reactor was then sealed and heated to about 80° C. VF2 monomer (0.408 kg) was charged into the reactor to a pressure of about 4,480 kPa. The reactor temperature stabilized at about 82° C. After the temperature stabilized, an aqueous solution (0.120 kg), containing the radical initiator potassium persulfate (3.50 wt. %) and the buffering agent sodium acetate (3.50 wt. %) was fed to the reactor. The reaction pressure was maintained at 4480 kPa by the addition of VF2 as needed. Additional amounts of initiator / buffer solution were added as follows during the reaction to maintain the reaction rate: 0.0100 L, after 0.804 kg of VF2 had been fed to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com