Patents
Literature
32 results about "Mri compatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
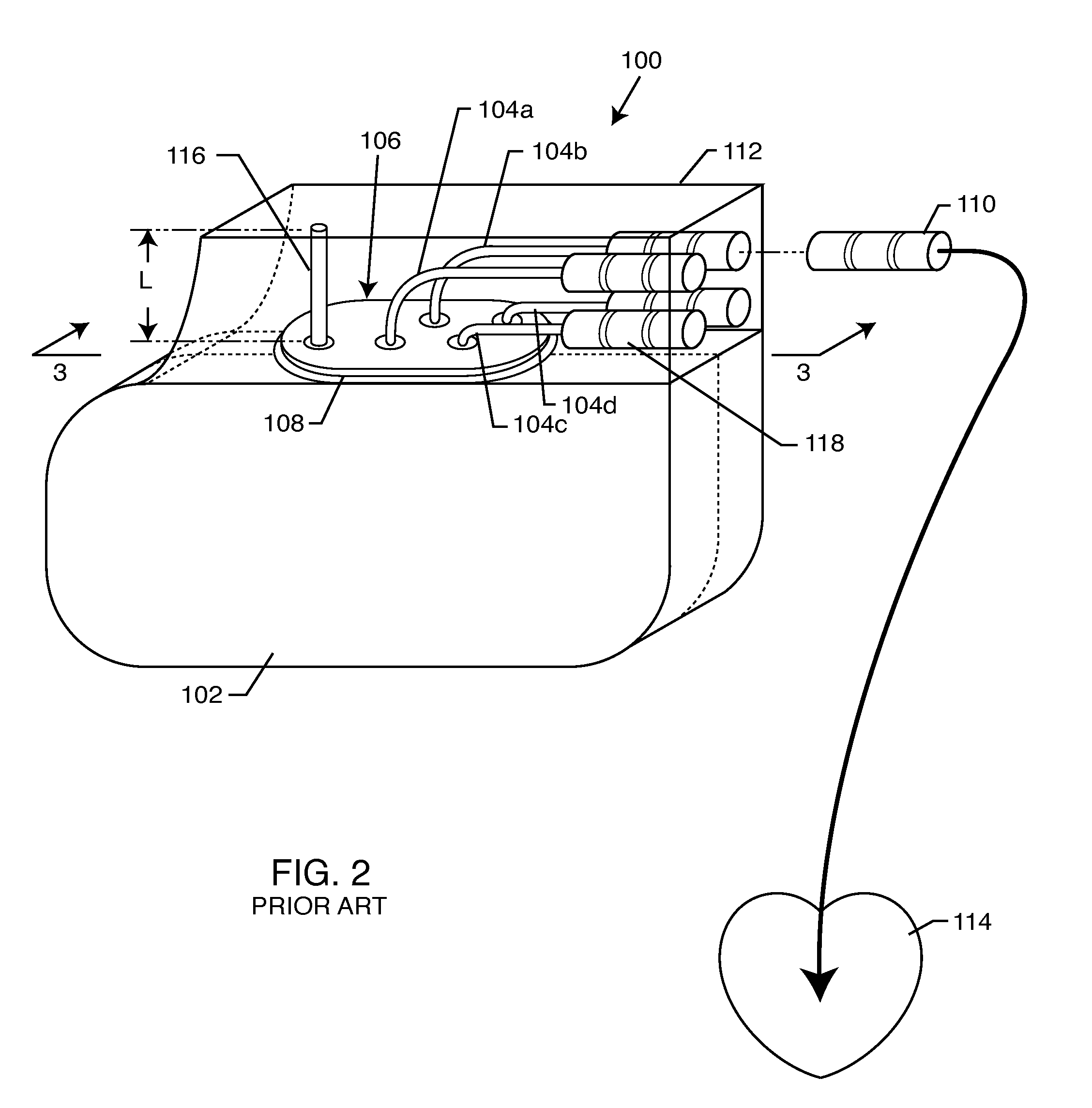
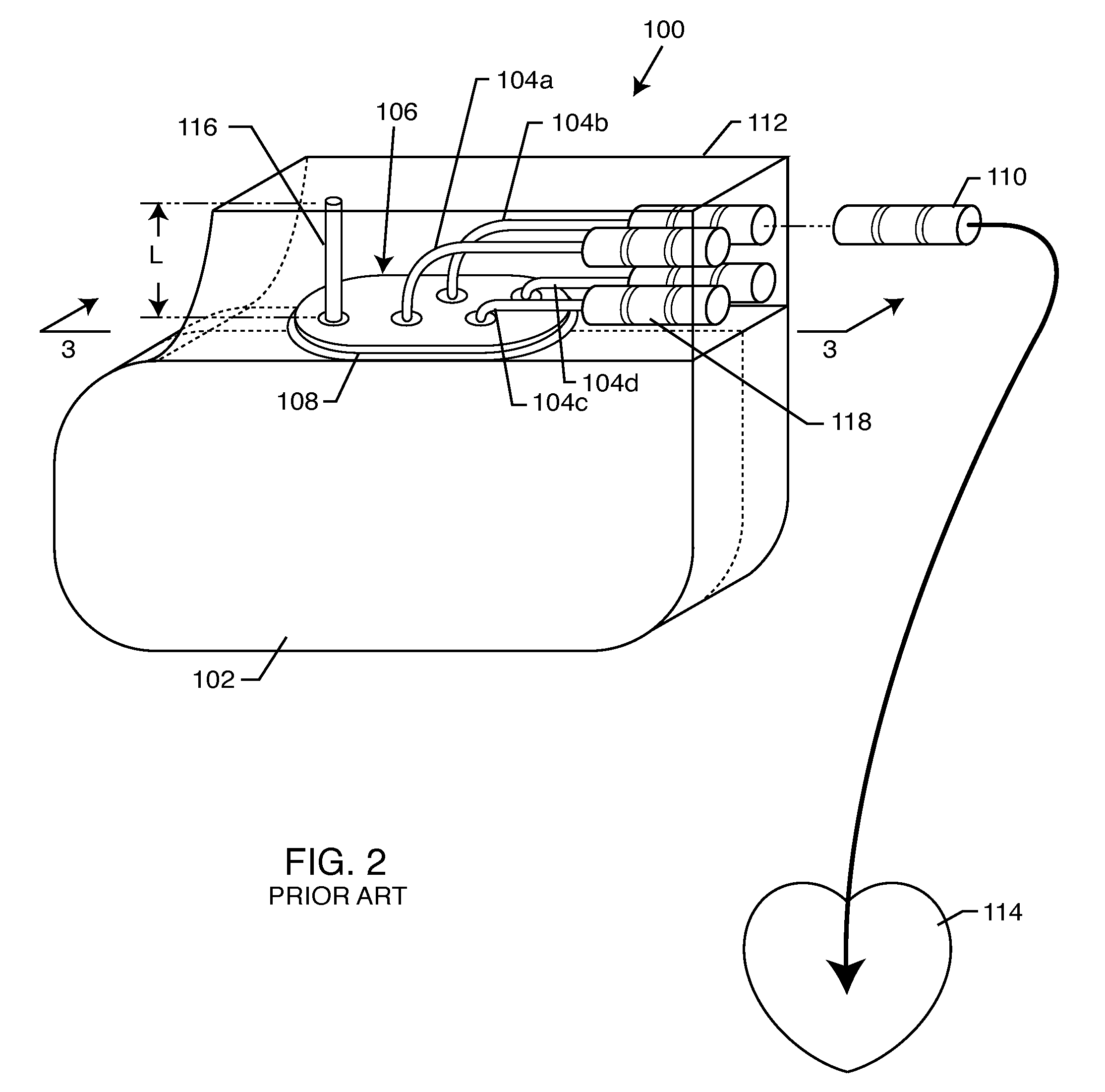
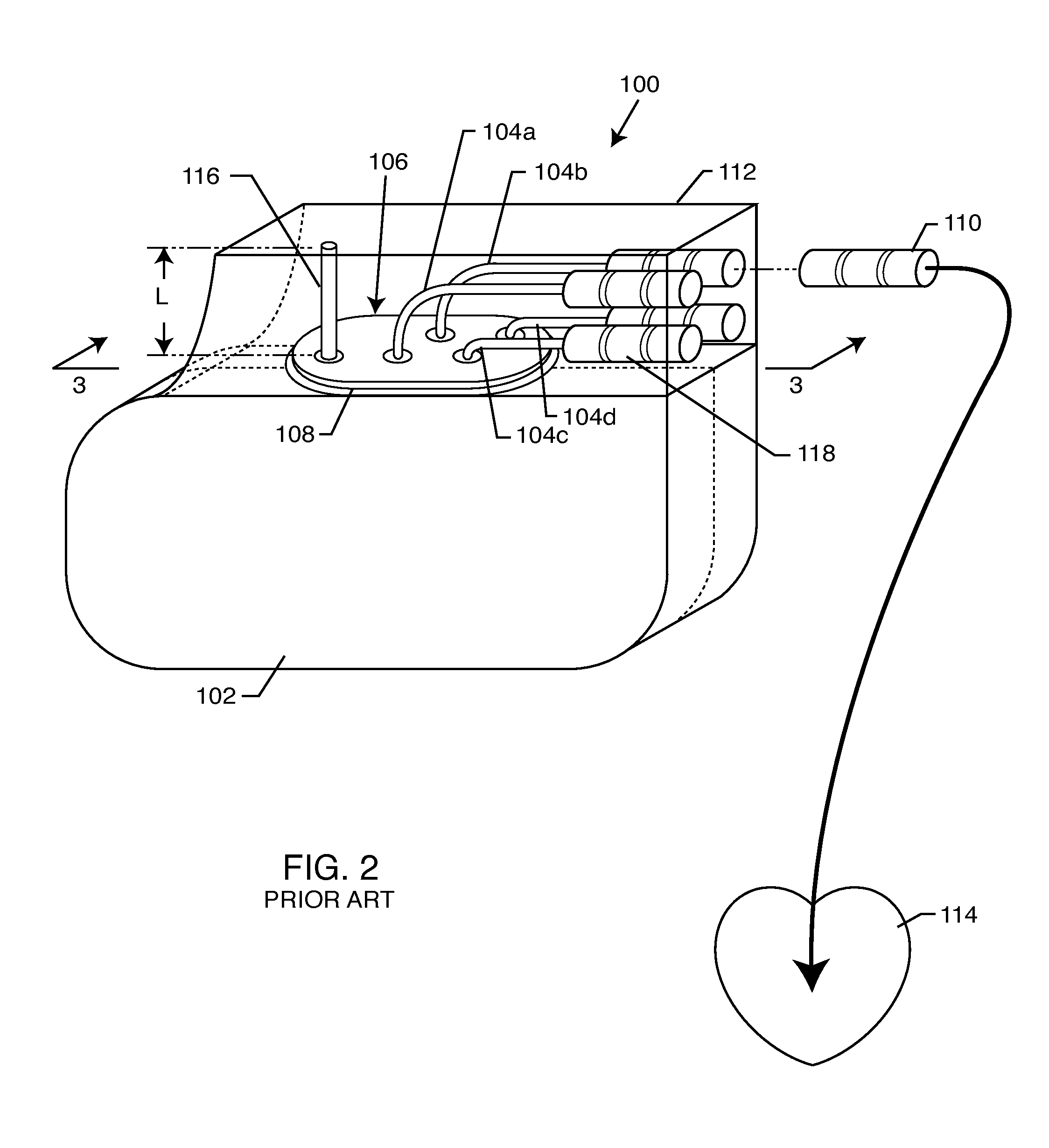
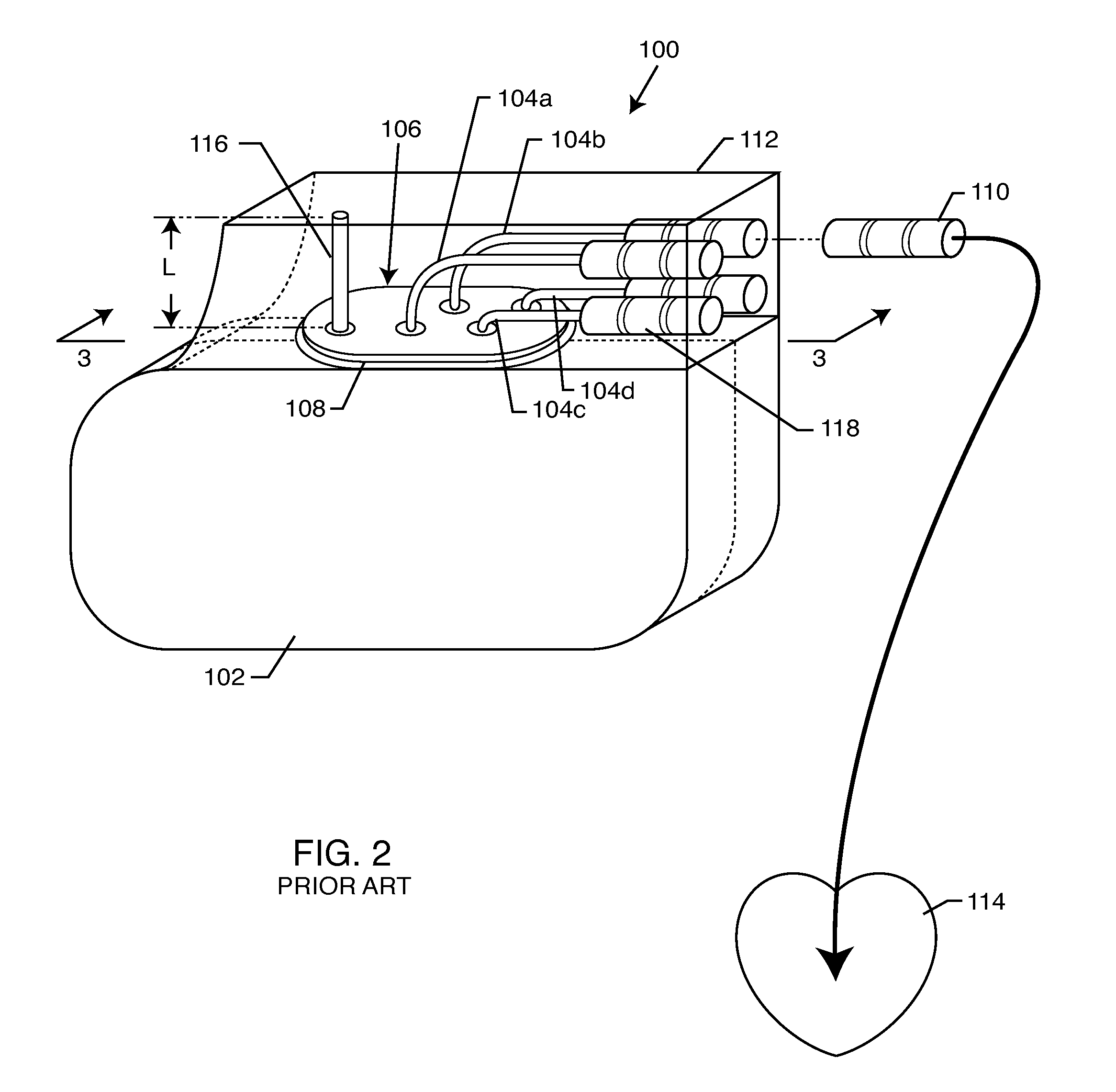
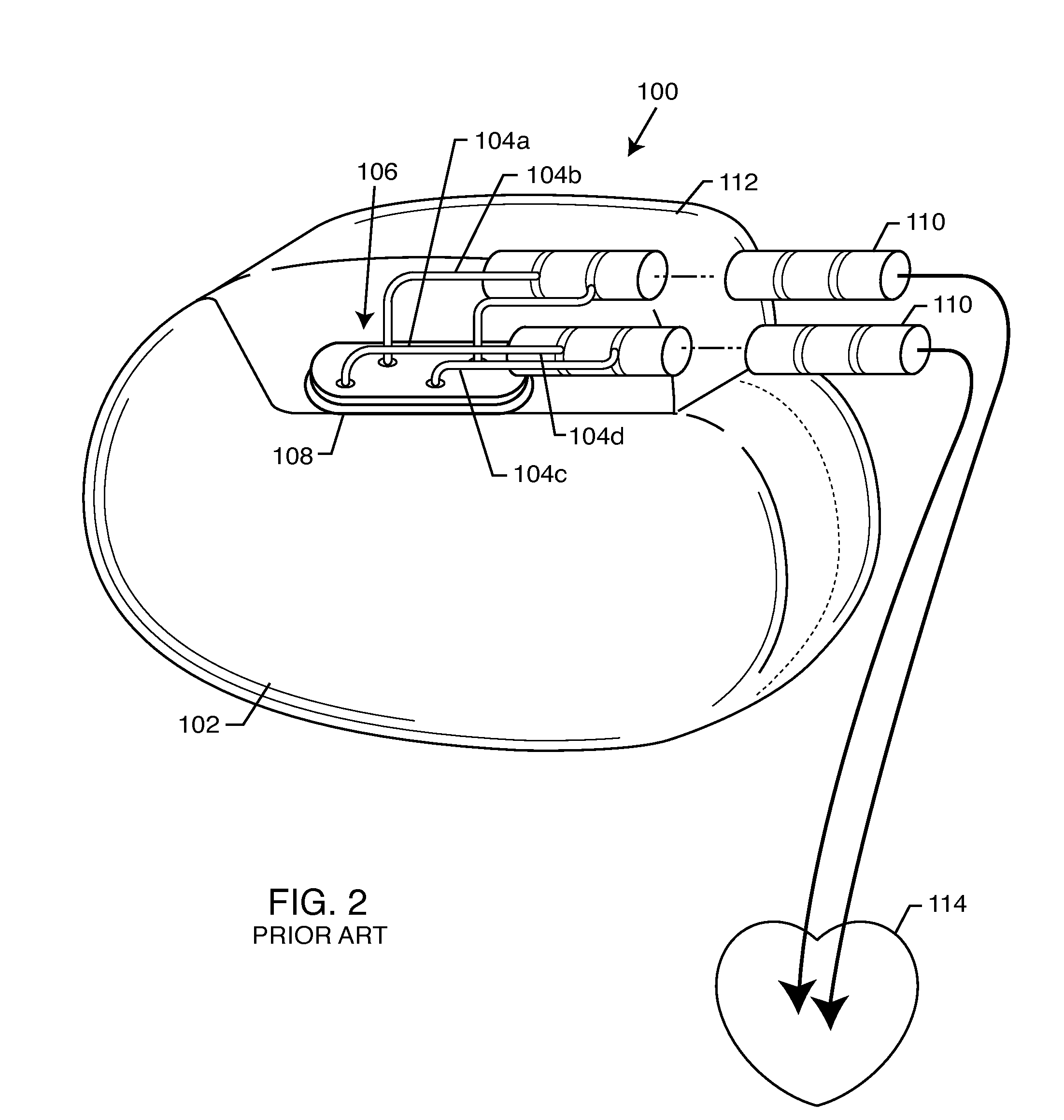
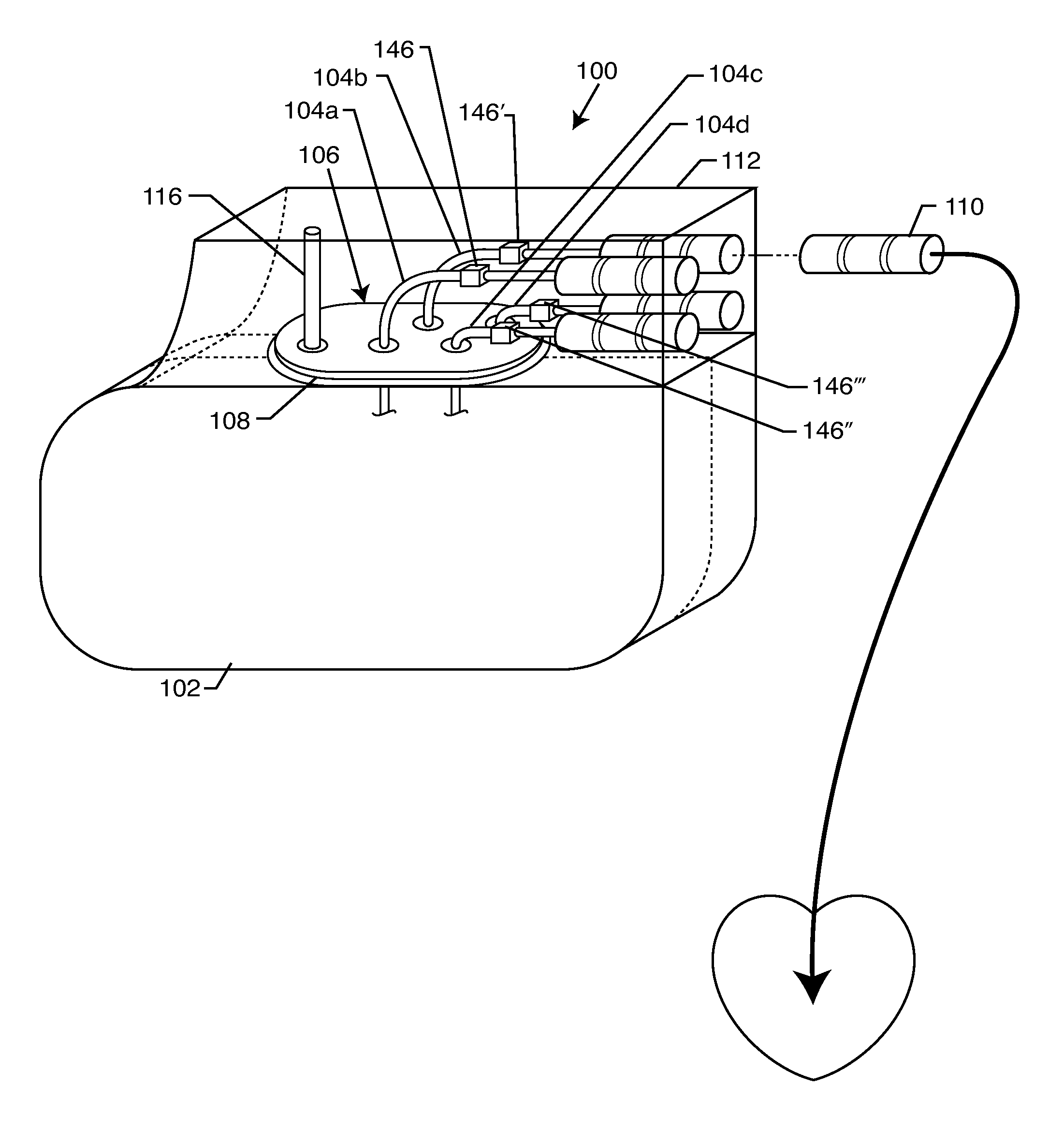
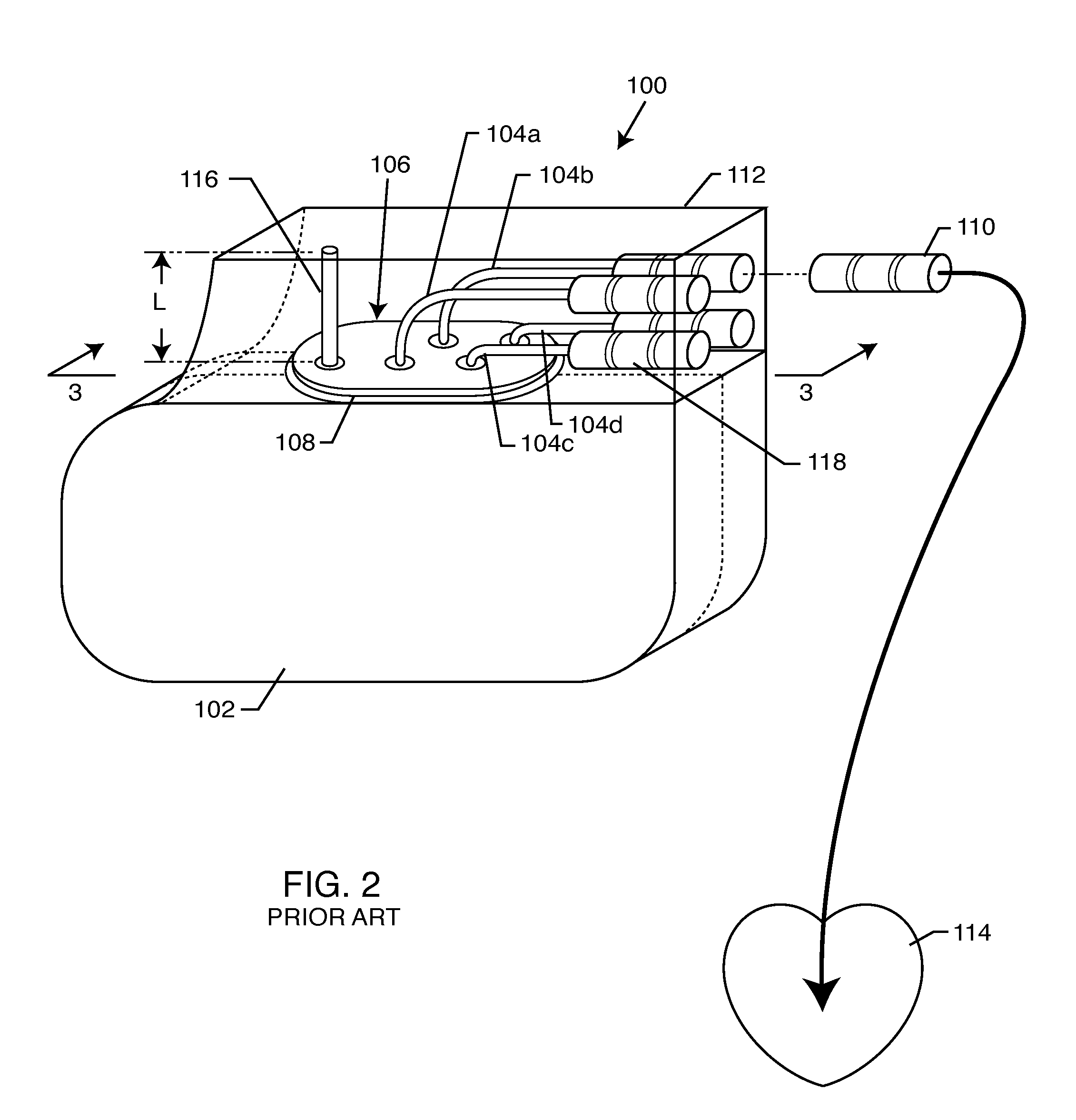
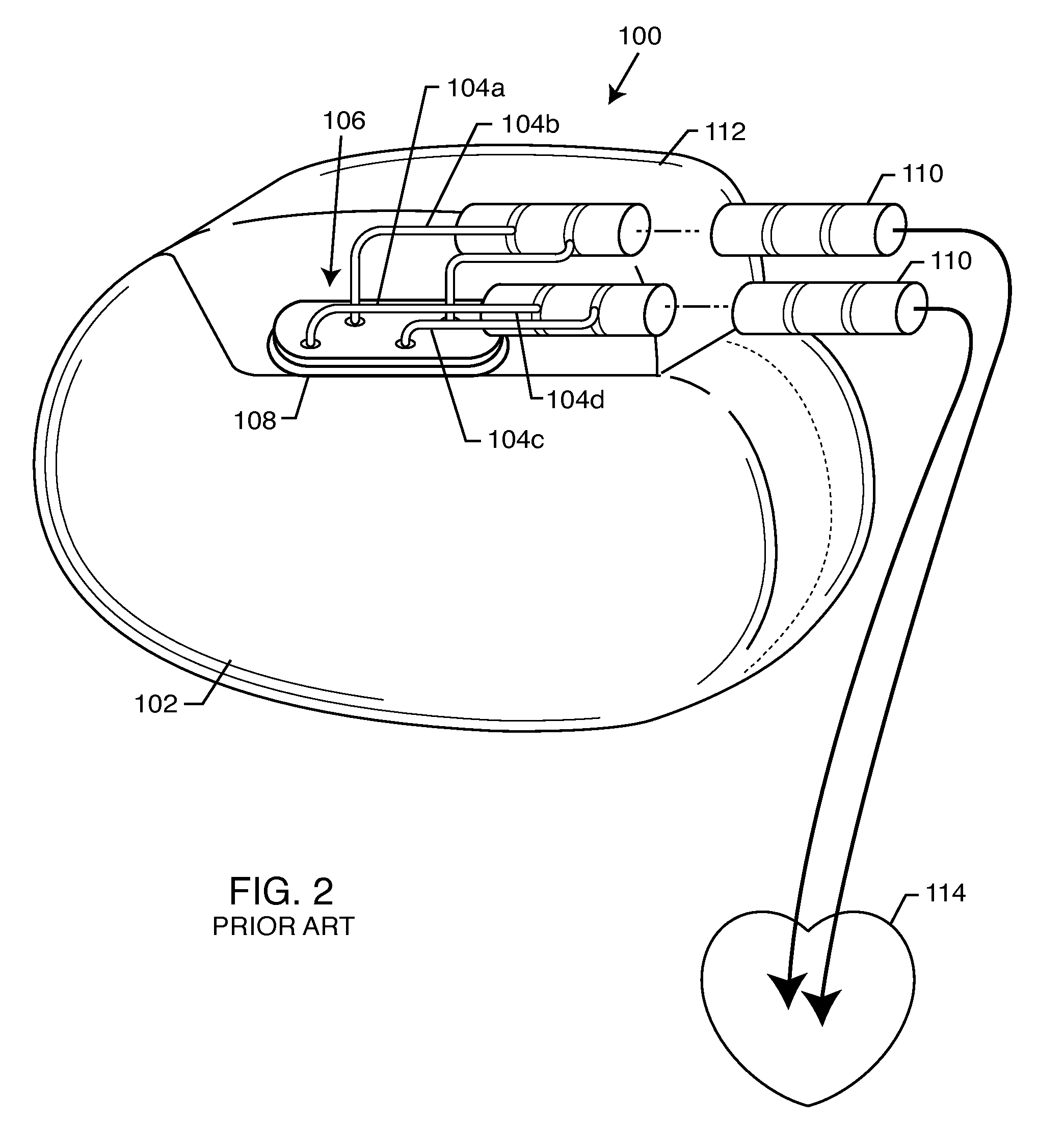
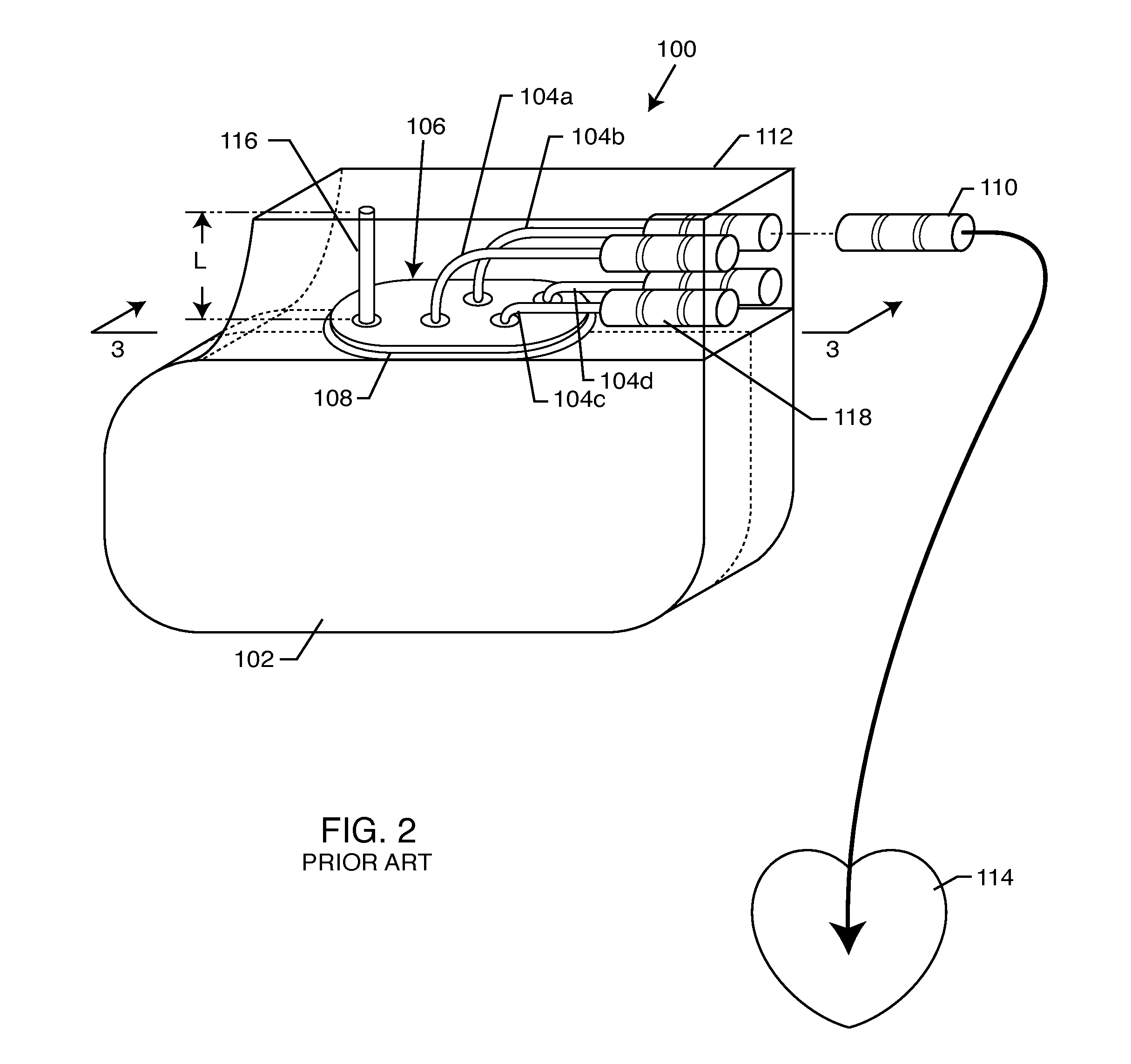
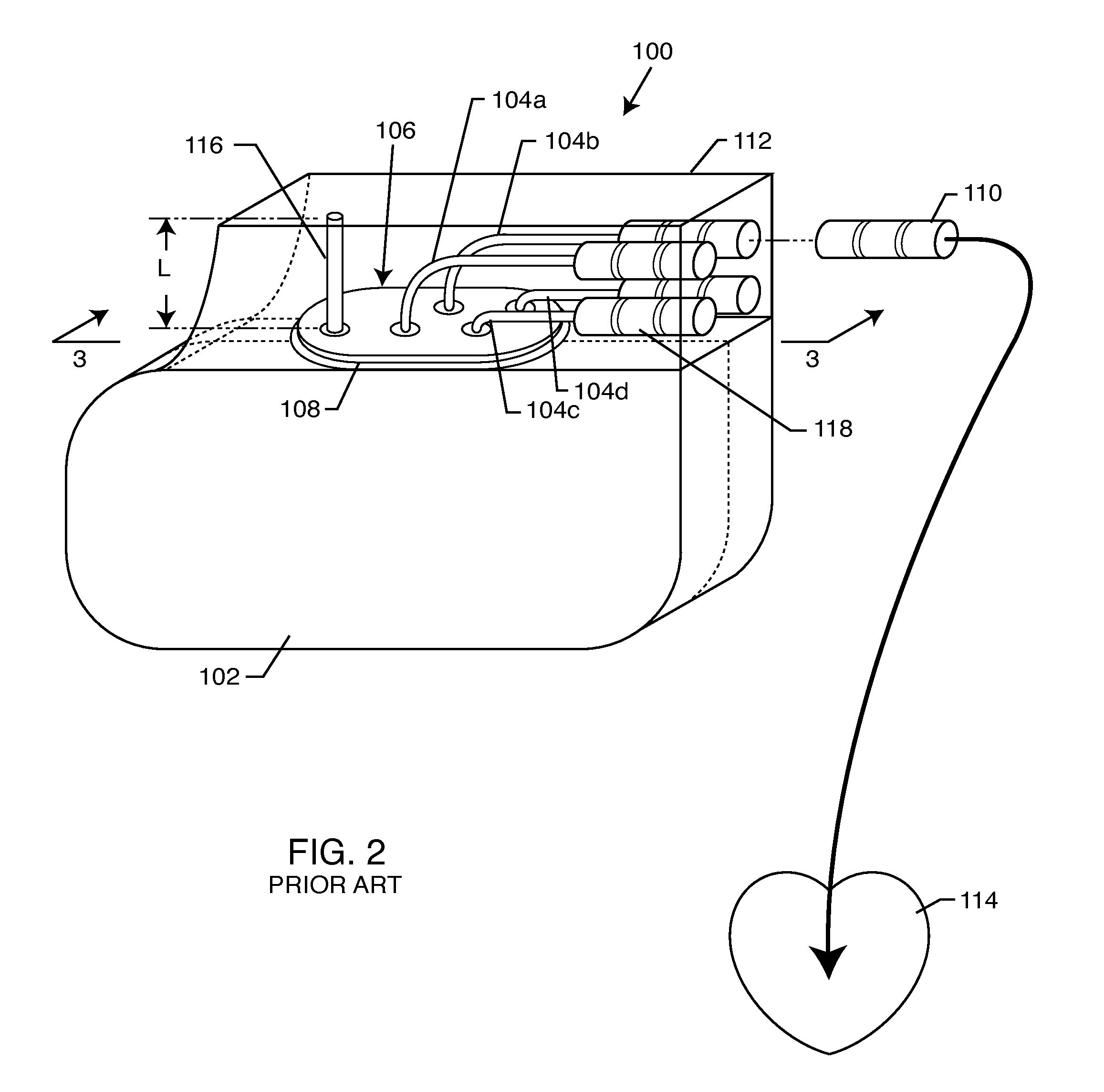
Non-ferromagnetic tank filters in lead wires of active implantable medical devices to enhance MRI compatibility
InactiveUS20080049376A1High impedanceMuch smaller and volumetrically efficientMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
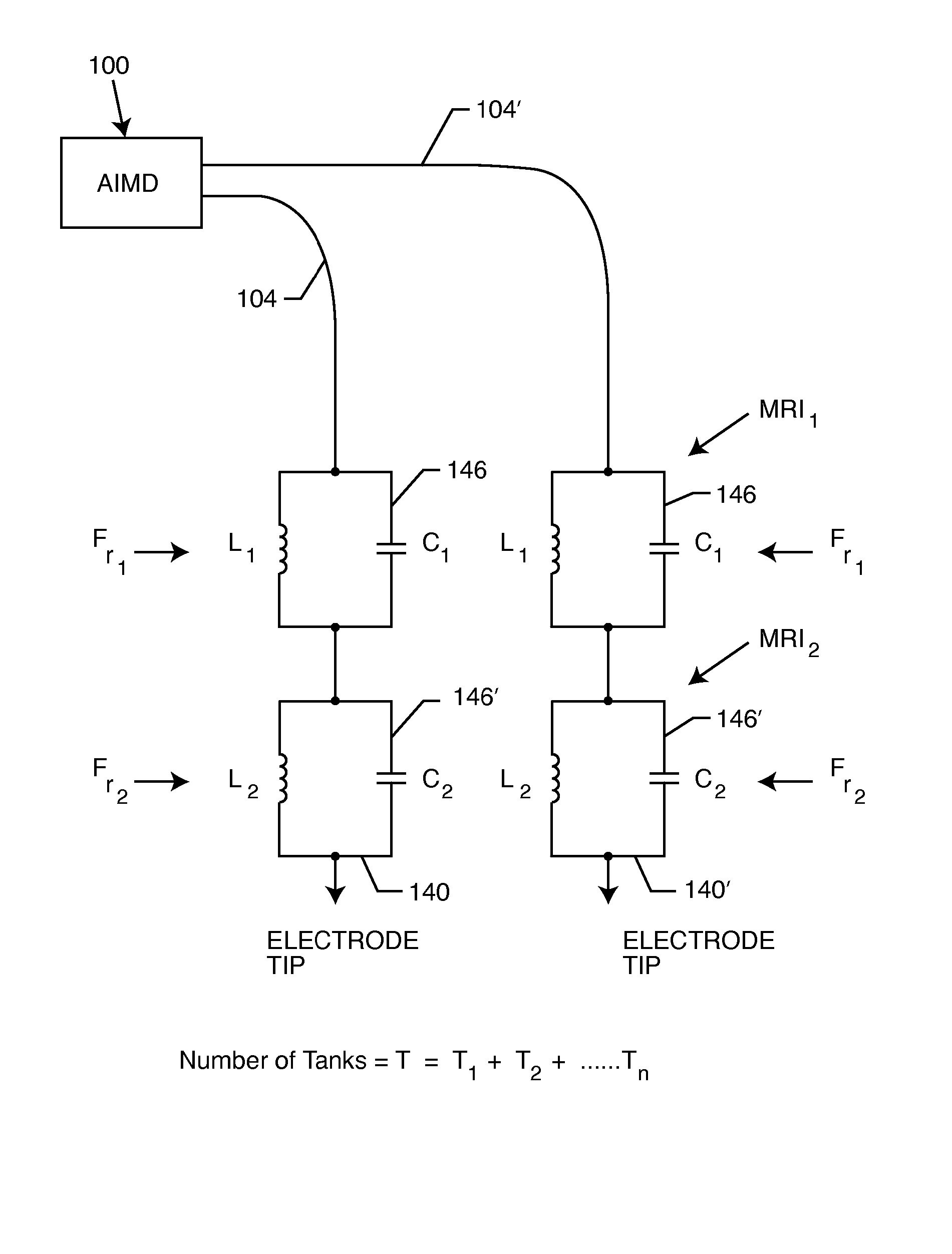
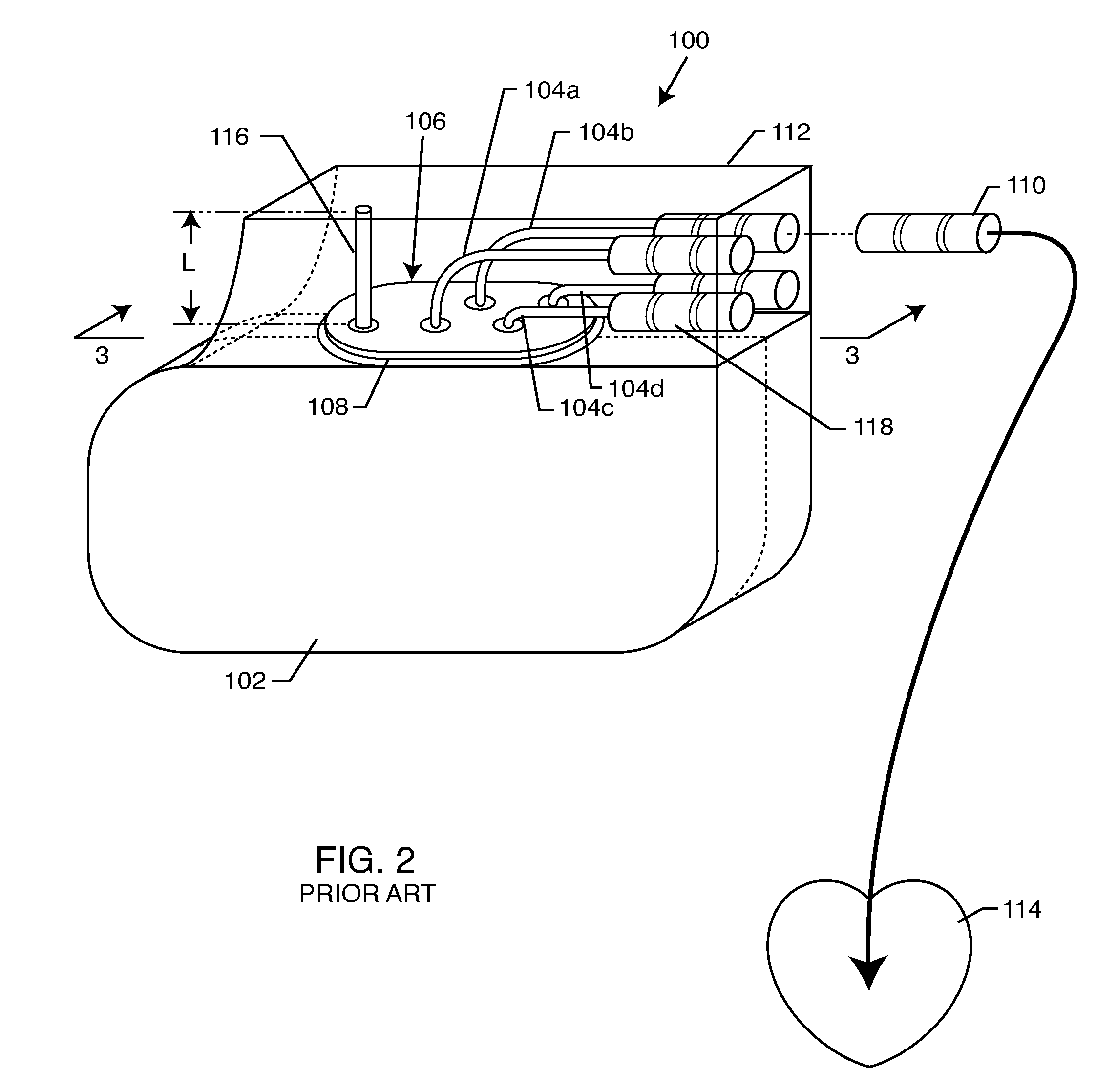
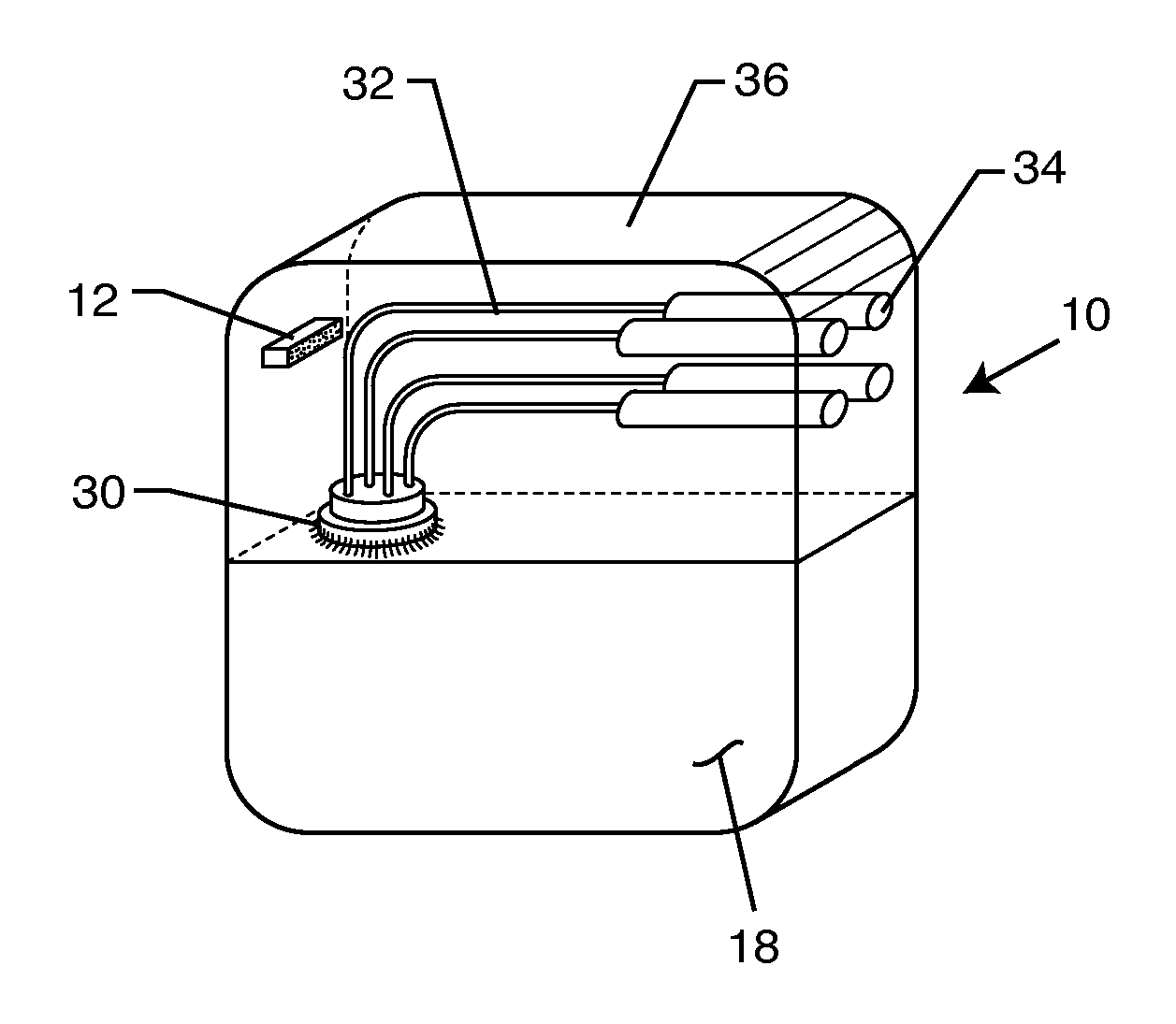
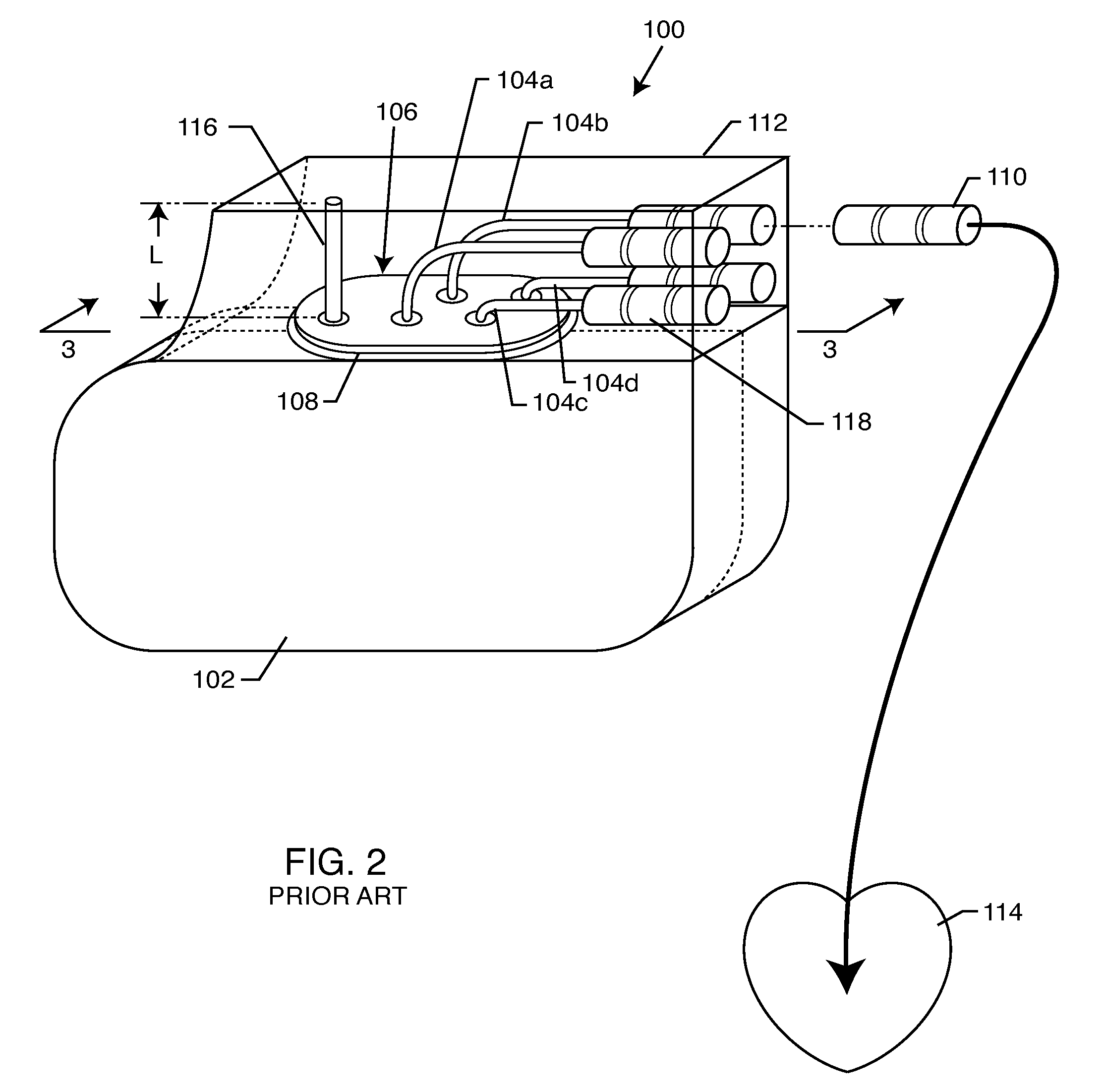
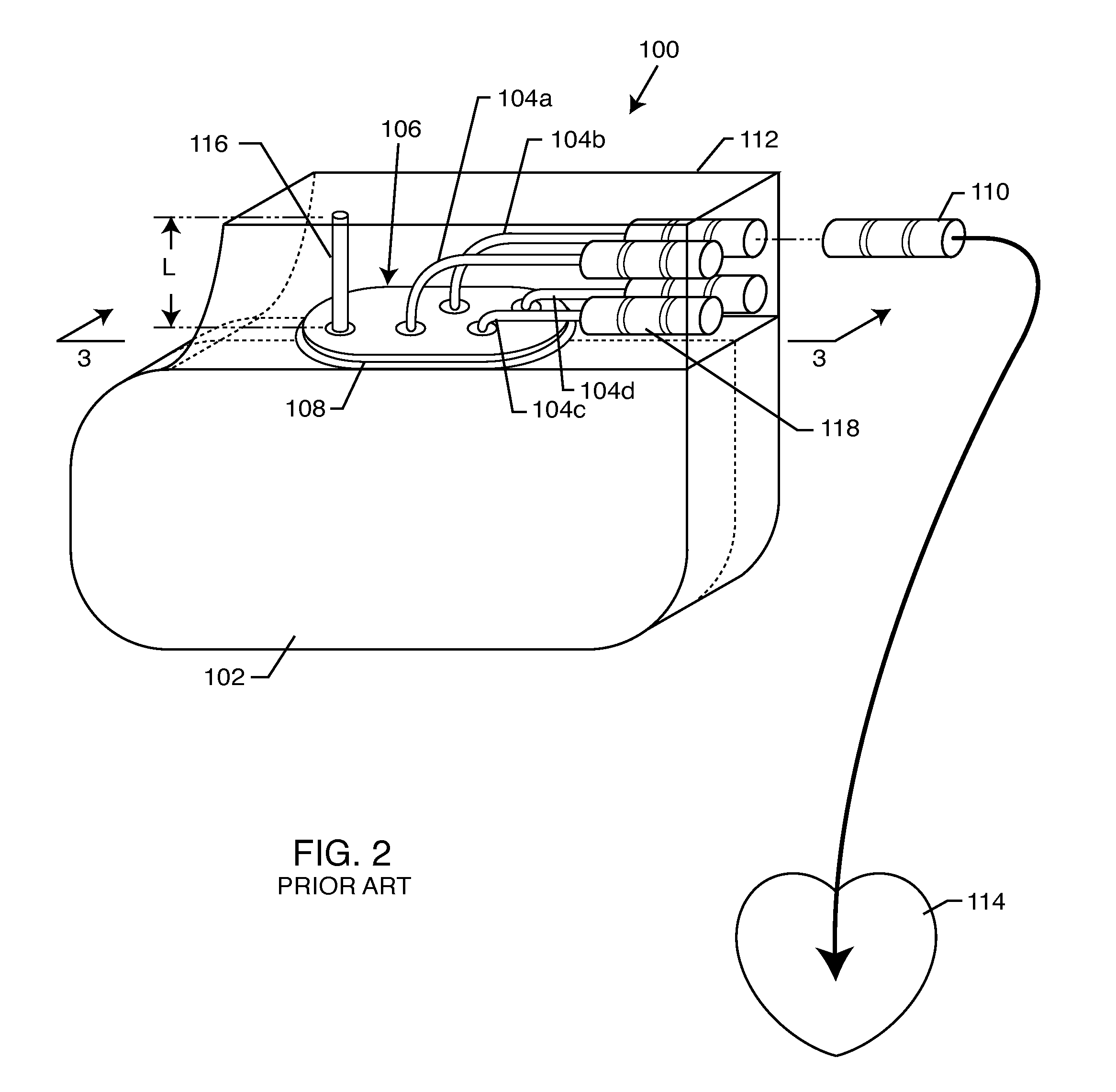
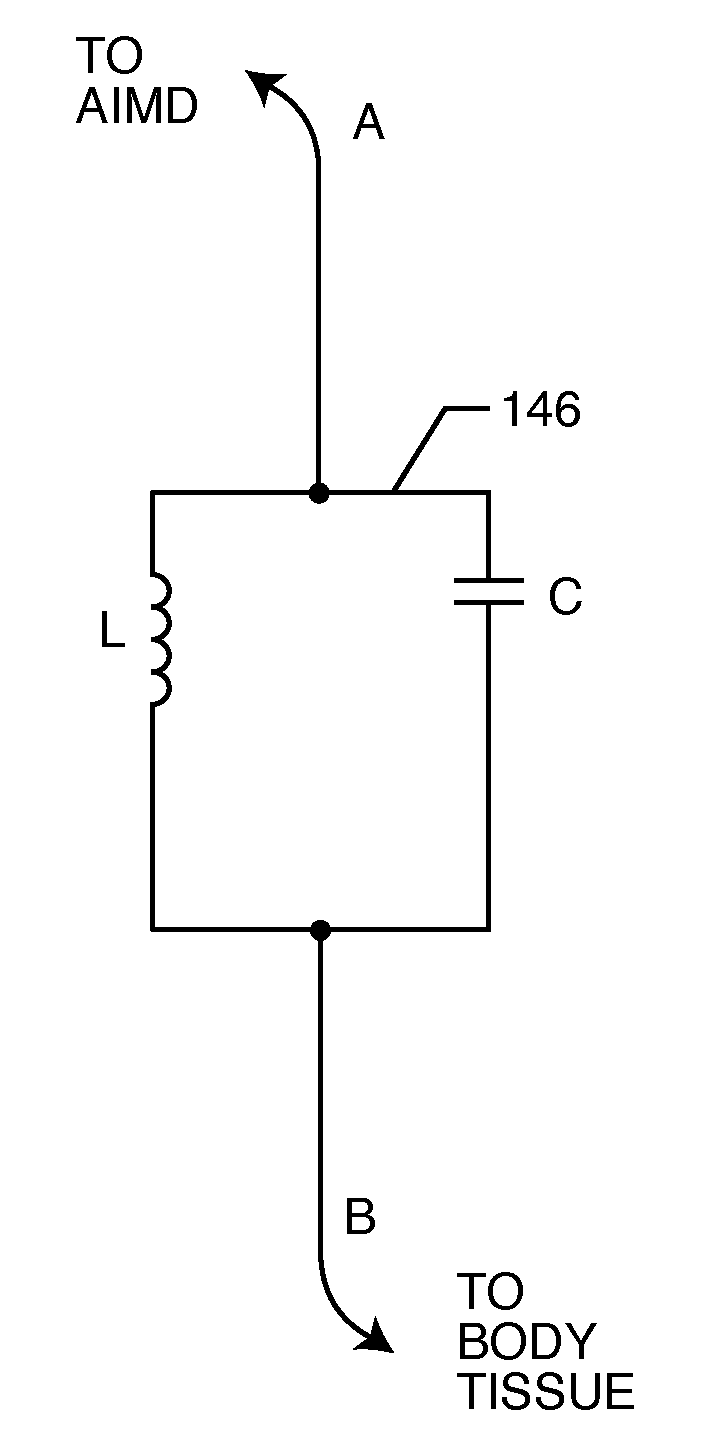
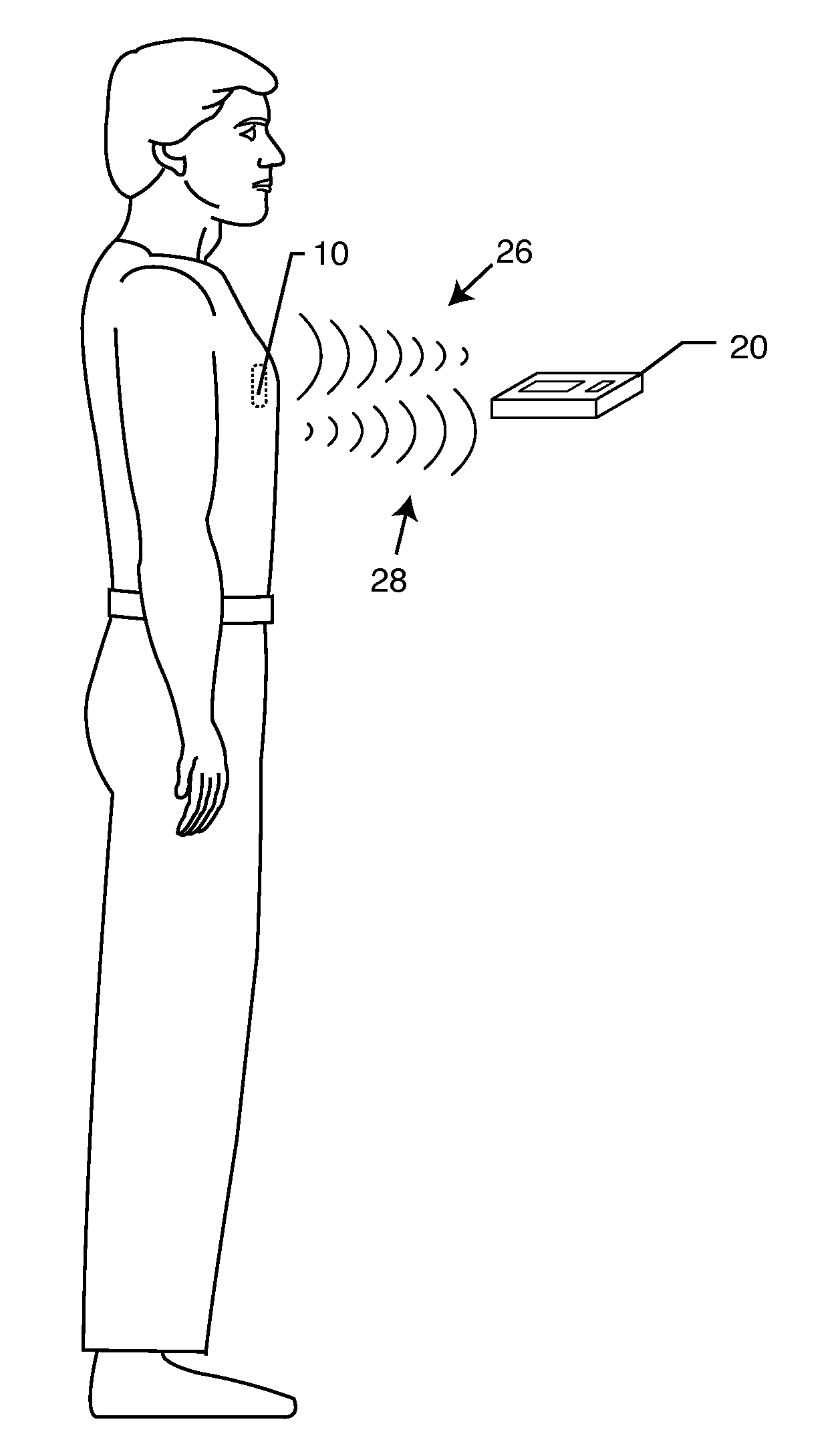
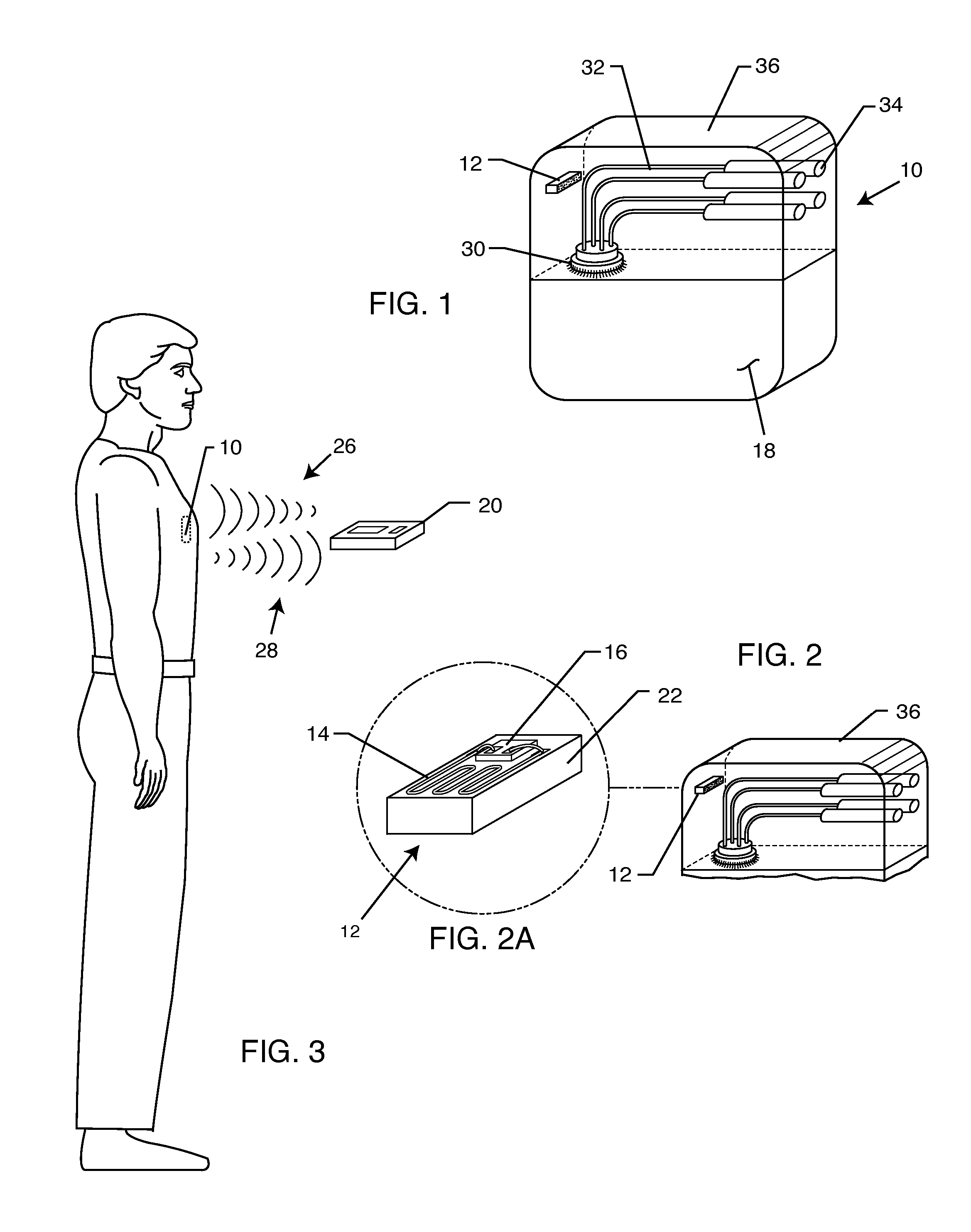
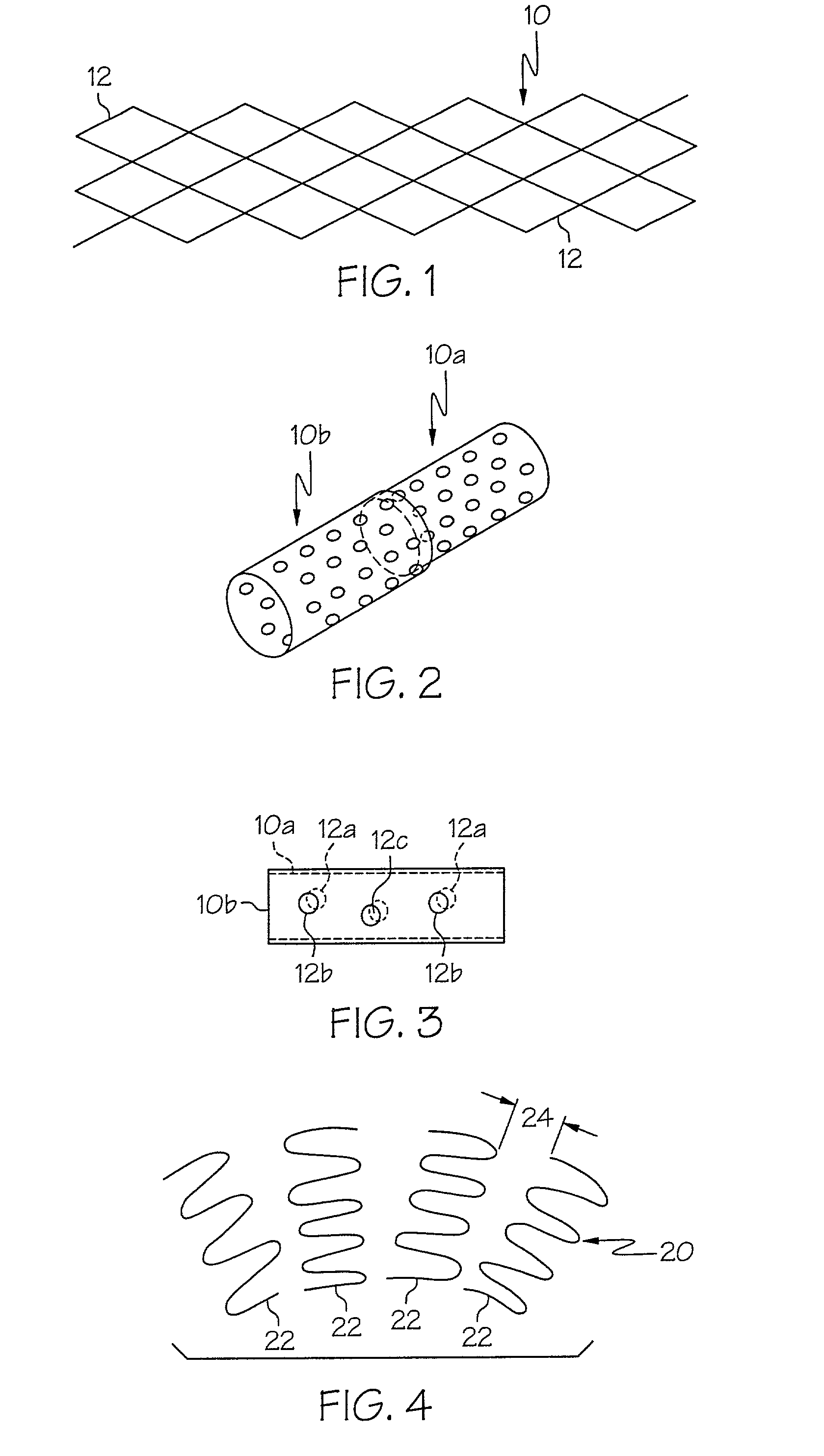
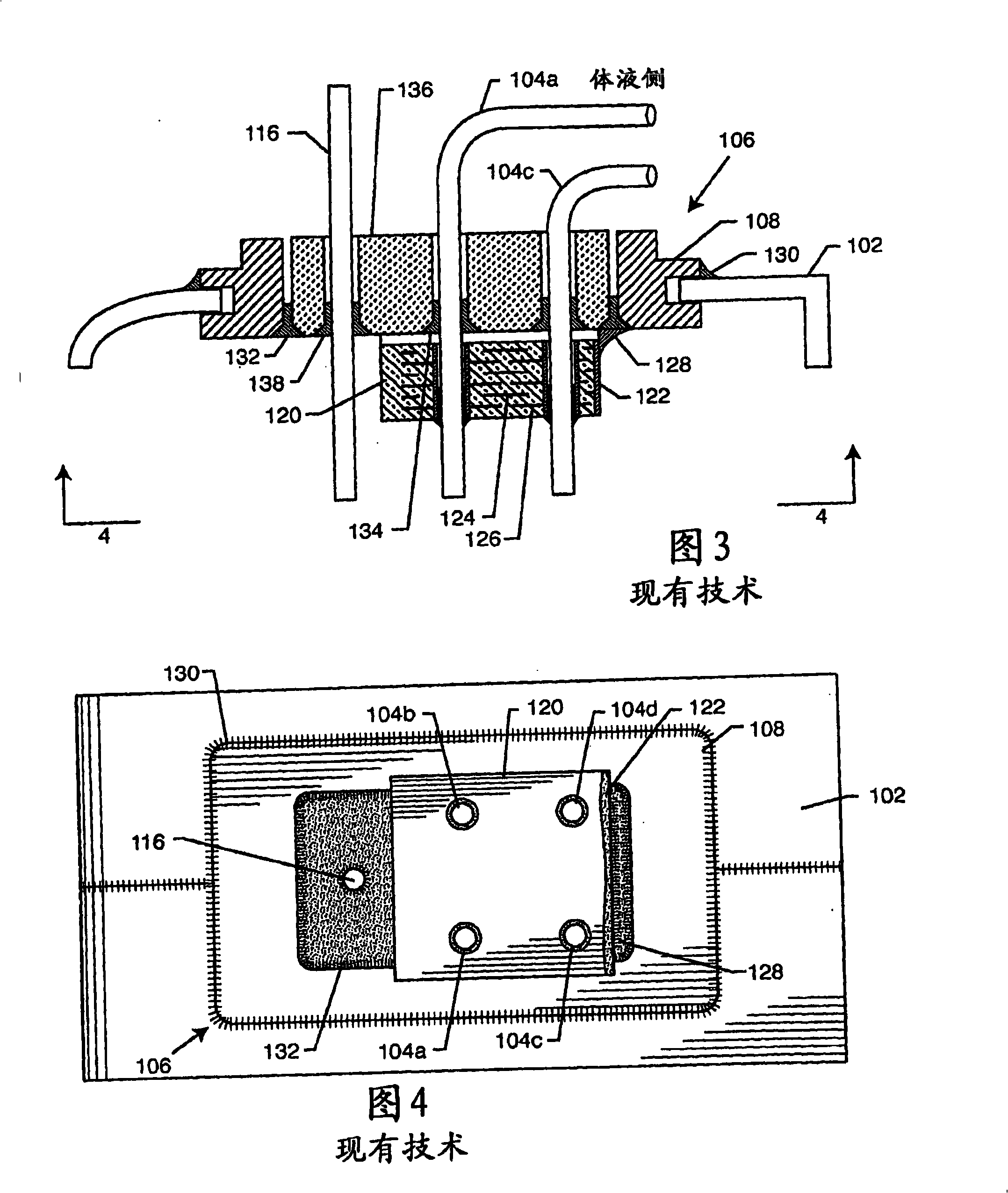
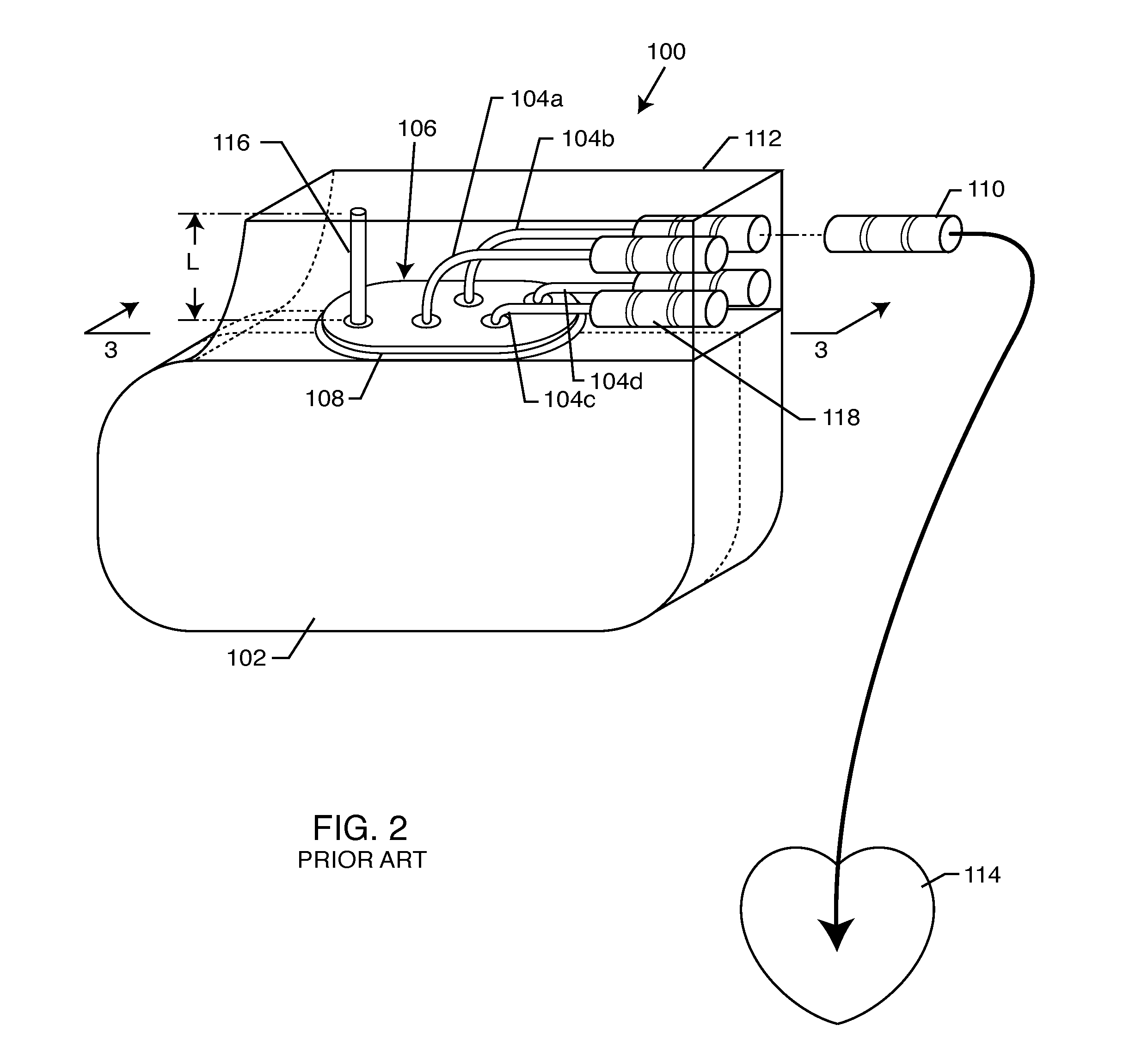
A TANK filter is provided for a lead wire of an active medical device (AMD). The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency. In a preferred form, the TANK filter reduces or even eliminates the use of ferro-magnetic materials, and instead uses non-ferromagnetic materials so as to reduce or eliminate MRI image artifacts or the force or torque otherwise associated during an MRI image scan.
Owner:WILSON GREATBATCH LTD
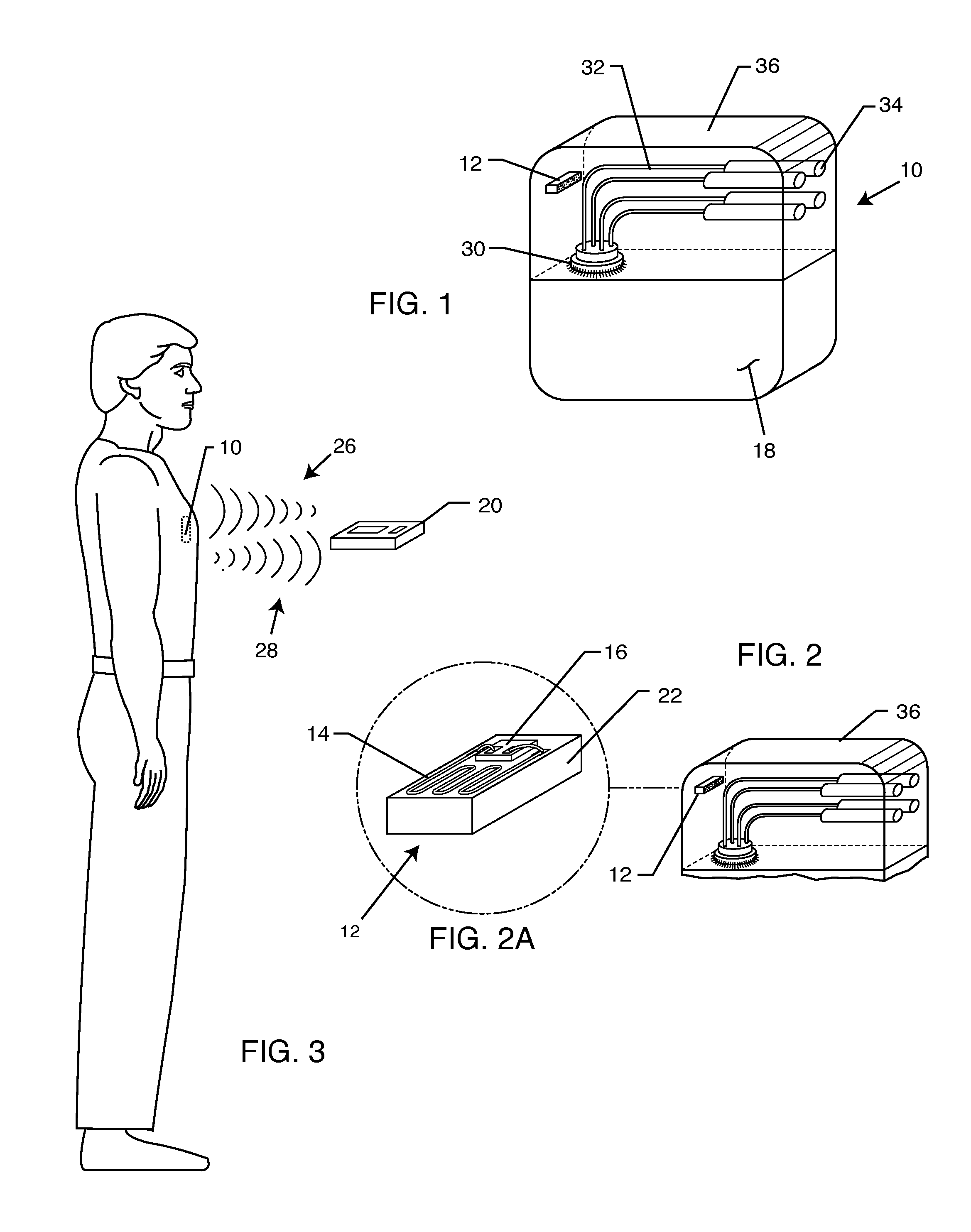
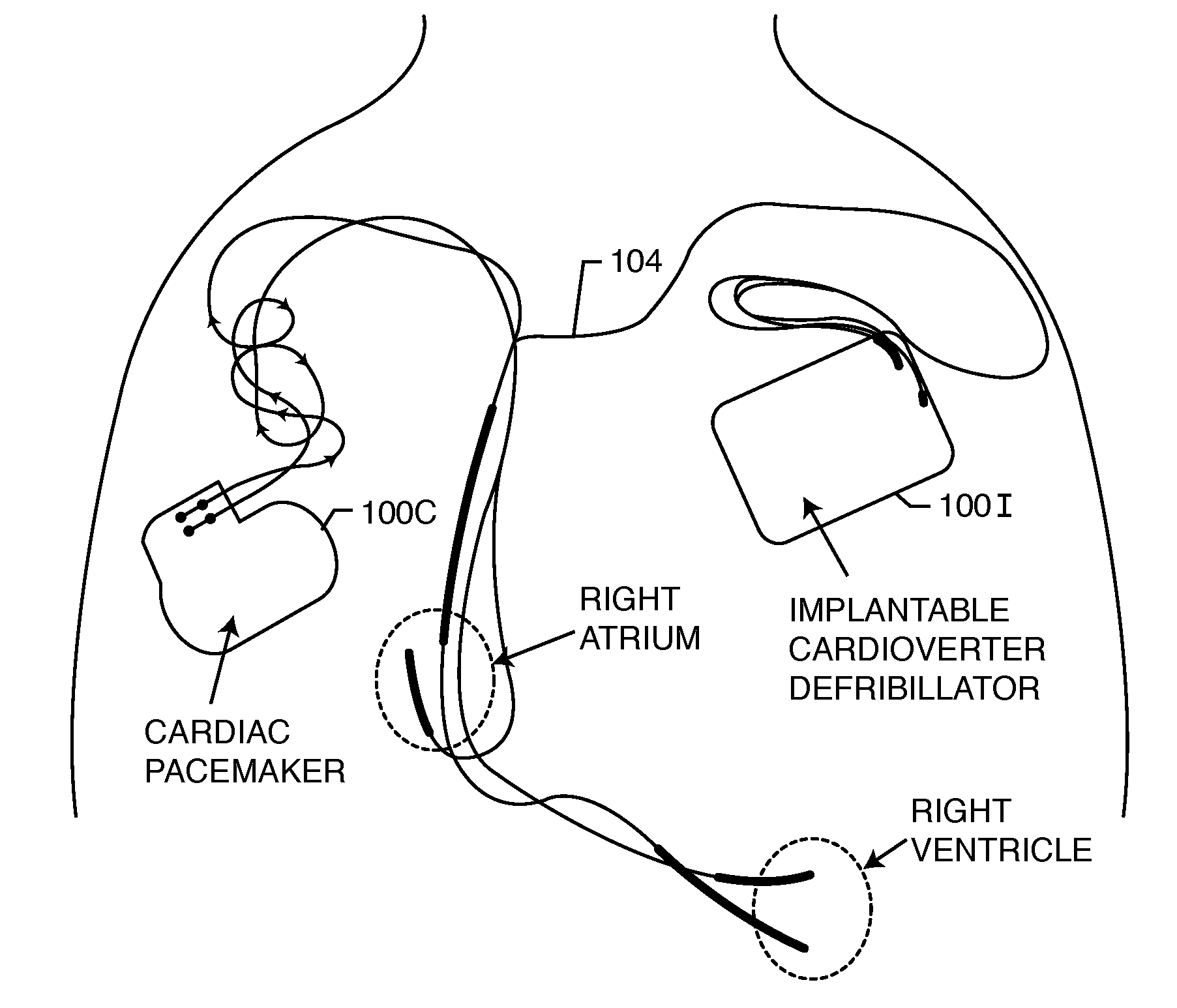
RFID detection and identification system for implantable medical lead systems
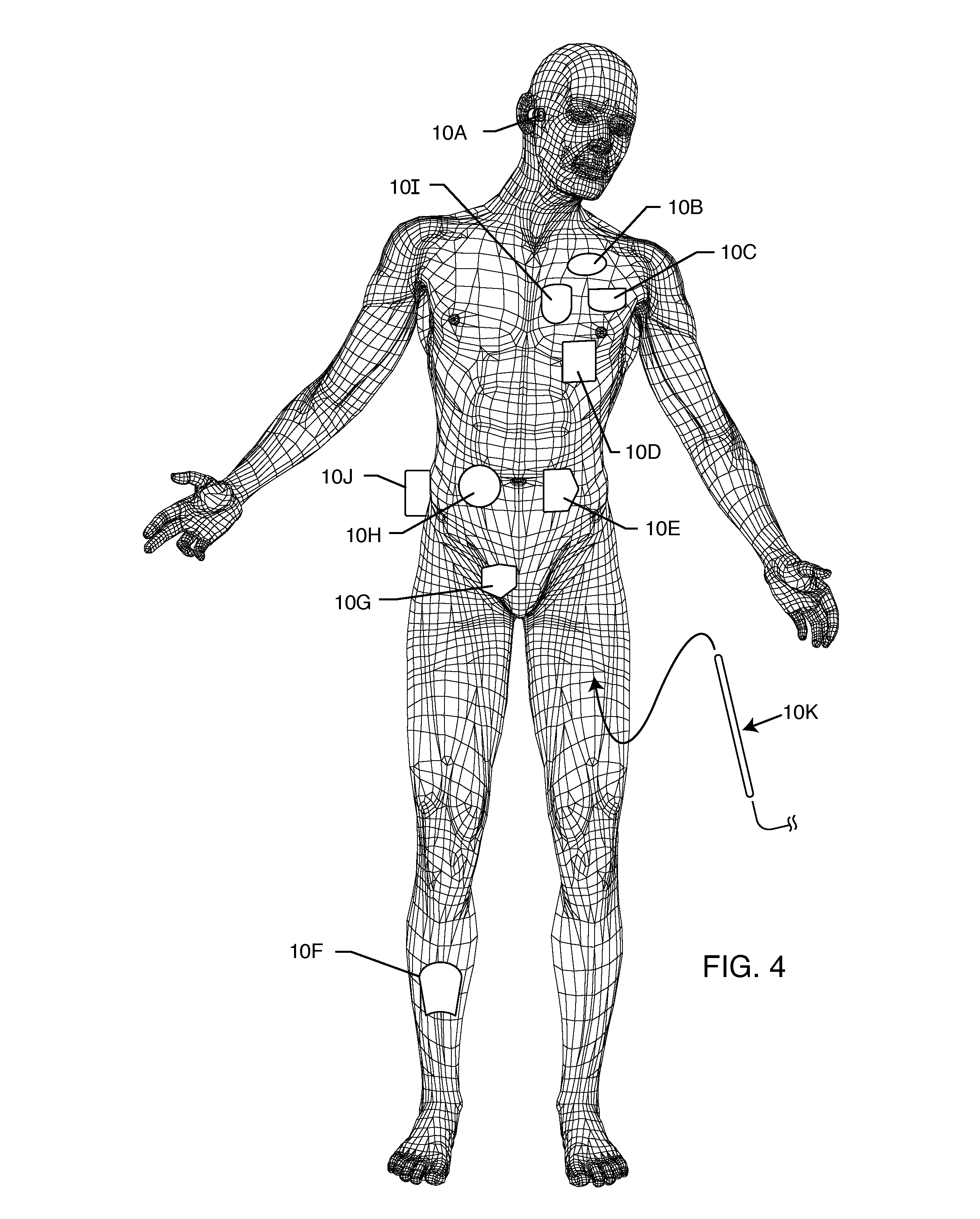
A system for identifying active implantable medical devices (AIMD) and lead systems implanted in a patient using a radio frequency identification (RFID) tag having retrievable information relating to the AIMD, lead system and / or patient. The RFID tag may store information about the AIMD manufacturer, model number, serial number; lead wire system placement information and manufacturer information; MRI compatibility due to the incorporation of bandstop filters; patient information, and physician and / or hospital information and other relevant information. The RFID tag may be affixed or disposed within the AIMD or lead wires of the lead system, or surgically implanted within a patient adjacent to the AIMD or lead wire system.
Owner:WILSON GREATBATCH LTD
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS20080161886A1Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsEngineeringInductor
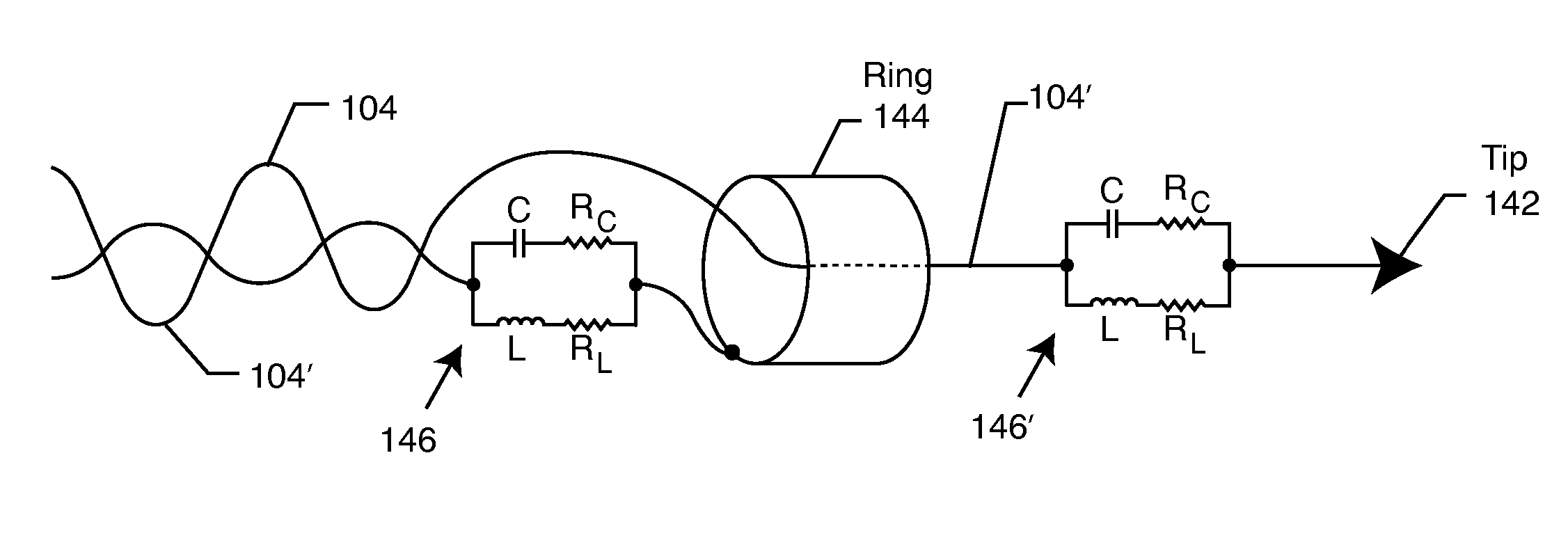
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Tank filters utilizing very low k materials, in series with lead wires or circuits of active medical devices to enhance MRI compatibility
ActiveUS20080071313A1Low dielectric constantHigh strengthMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A TANK filter is provided for a lead wire of an active medical device (AMD). In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device. The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency to attenuate current flow through the lead wire along a range of selected frequencies. In a particularly preferred form, the TANK filter is manufactured using very low k materials of sufficient strength to handle forces applied thereto during installation and use.
Owner:WILSON GREATBATCH LTD
Band stop filter employing a capacitor and an inductor tank circuit to enhance MRI compatibility of active medical devices
InactiveUS20060247684A1Decrease QCapacitor is relatively minimizedMultiple-port networksInternal electrodesCapacitanceEngineering
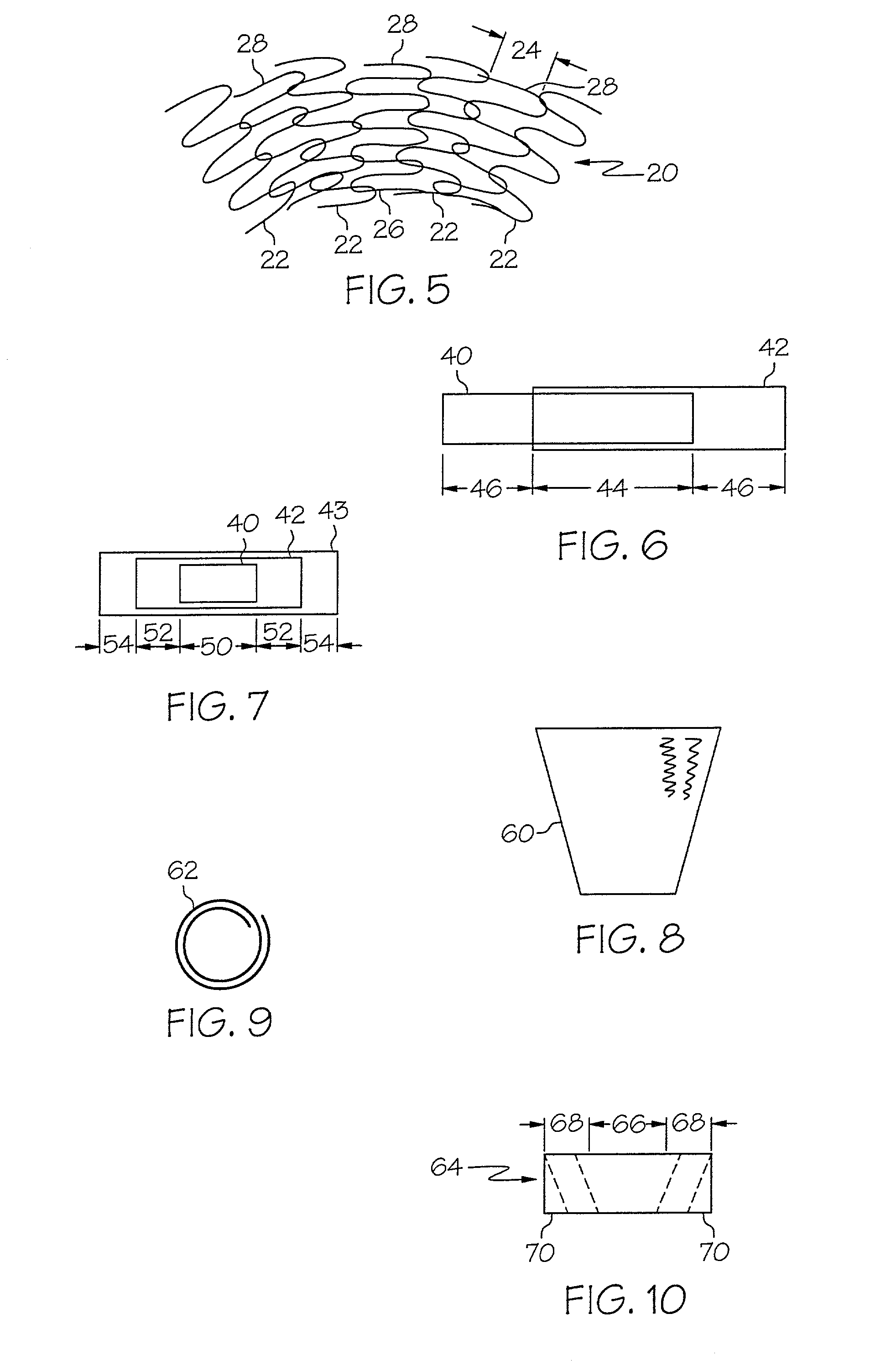
A band stop filter is provided for a lead wire of an active medical device (AMD). The band stop filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the band stop filter is resonant at a selected frequency. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the band stop filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the band stop filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Band stop filter employing a capacitor and an inductor tank circuit to enhance MRI compatibility of active implantable medical devices
InactiveUS20070288058A1Avoid flowAvoid loopsMultiple-port networksInternal electrodesCapacitanceInductor
Owner:WILSON GREATBATCH LTD
Metal alloy for medical devices and implants
InactiveUS20080312740A1Maintain good propertiesImprove uniformityStentsHeart valvesNiobiumBiocompatibility Testing
Owner:ABBOTT IRELAND
Medical implant or device
The present invention relates to a medical device or implant made at least in part of a high strength, low modulus metal alloy comprising Niobium, Tantalum, and at least one element selected from the group consisting of Zirconium, Tungsten and Molybdenum. The medical devices according to the present invention provide superior characteristics with regard to biocompatibility, radio-opacity and MRI compatibility.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS7702387B2Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Implantable lead bandstop filter employing an inductive coil with parasitic capacitance to enhance MRI compatibility of active medical devices
InactiveUS8145324B1Maximized (or minimizedMinimize resistance lossMultiple-port networksSpinal electrodesParasitic capacitanceEngineering
A medical lead system includes at least one bandstop filter for attenuating current flow through the lead across a range of frequencies. The bandstop filter has an overall circuit Q wherein the resultant 3 dB bandwidth is at least 10 kHz. The values of capacitance and inductance of the bandstop filter are selected such that the bandstop filter is resonant at a selected center frequency or range of frequencies. Preferably, the bandstop filter has an overall circuit Q wherein the resultant 10 dB bandwidth is at least 10 kHz. Such bandstop filters are backwards compatible with known implantable deployment systems and extraction systems.
Owner:WILSON GREATBATCH LTD
RFID detection and identification system for implantable medical lead systems
A system for identifying active implantable medical devices (AIMD) and lead systems implanted in a patient using a radio frequency identification (RFID) tag having retrievable information relating to the AIMD, lead system and / or patient. The RFID tag may store information about the AIMD manufacturer, model number, serial number; lead wire system placement information and manufacturer information; MRI compatibility due to the incorporation of bandstop filters; patient information, and physician and / or hospital information and other relevant information. The RFID tag may be affixed or disposed within the AIMD or lead wires of the lead system, or surgically implanted within a patient adjacent to the AIMD or lead wire system.
Owner:WILSON GREATBATCH LTD
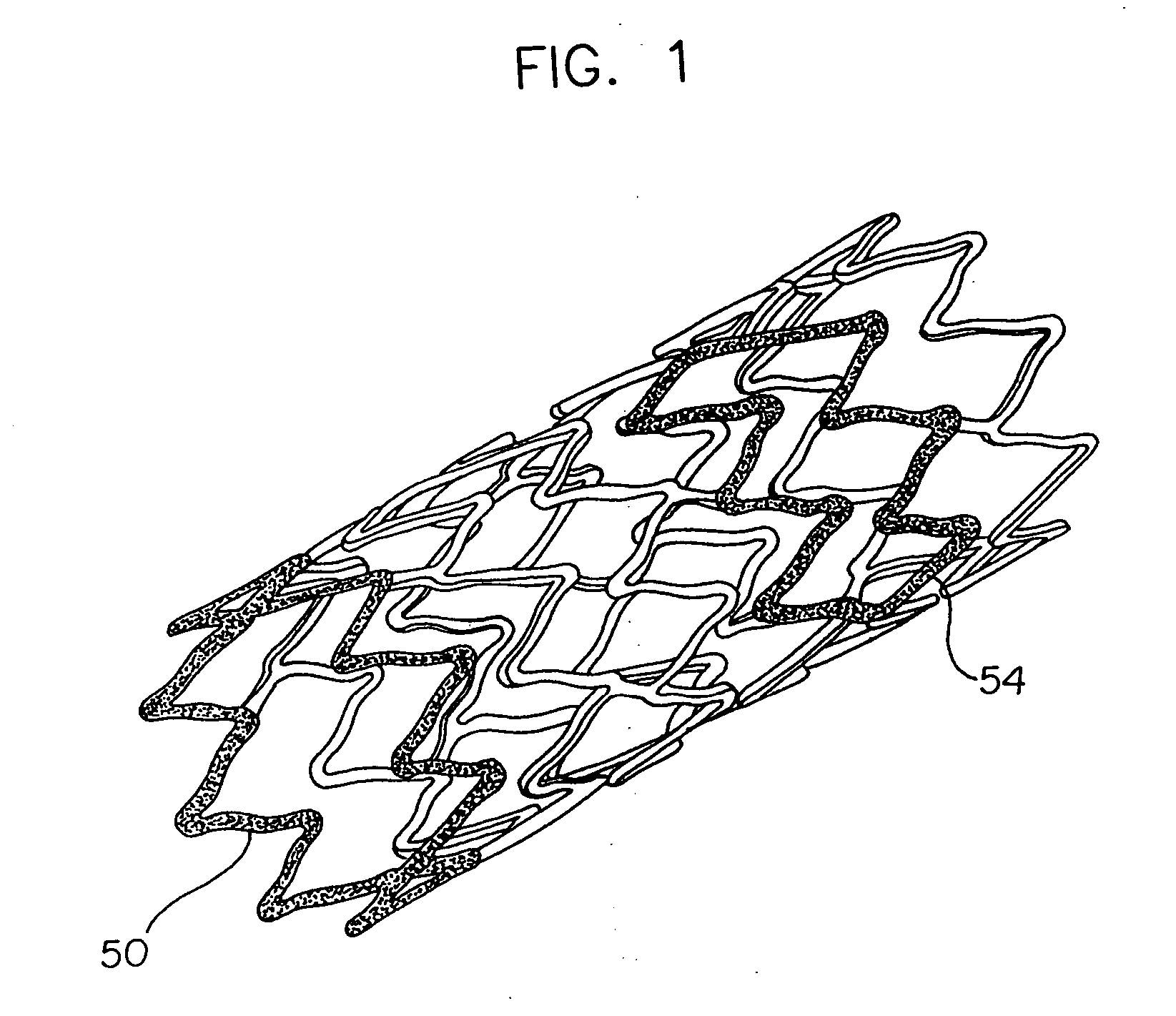
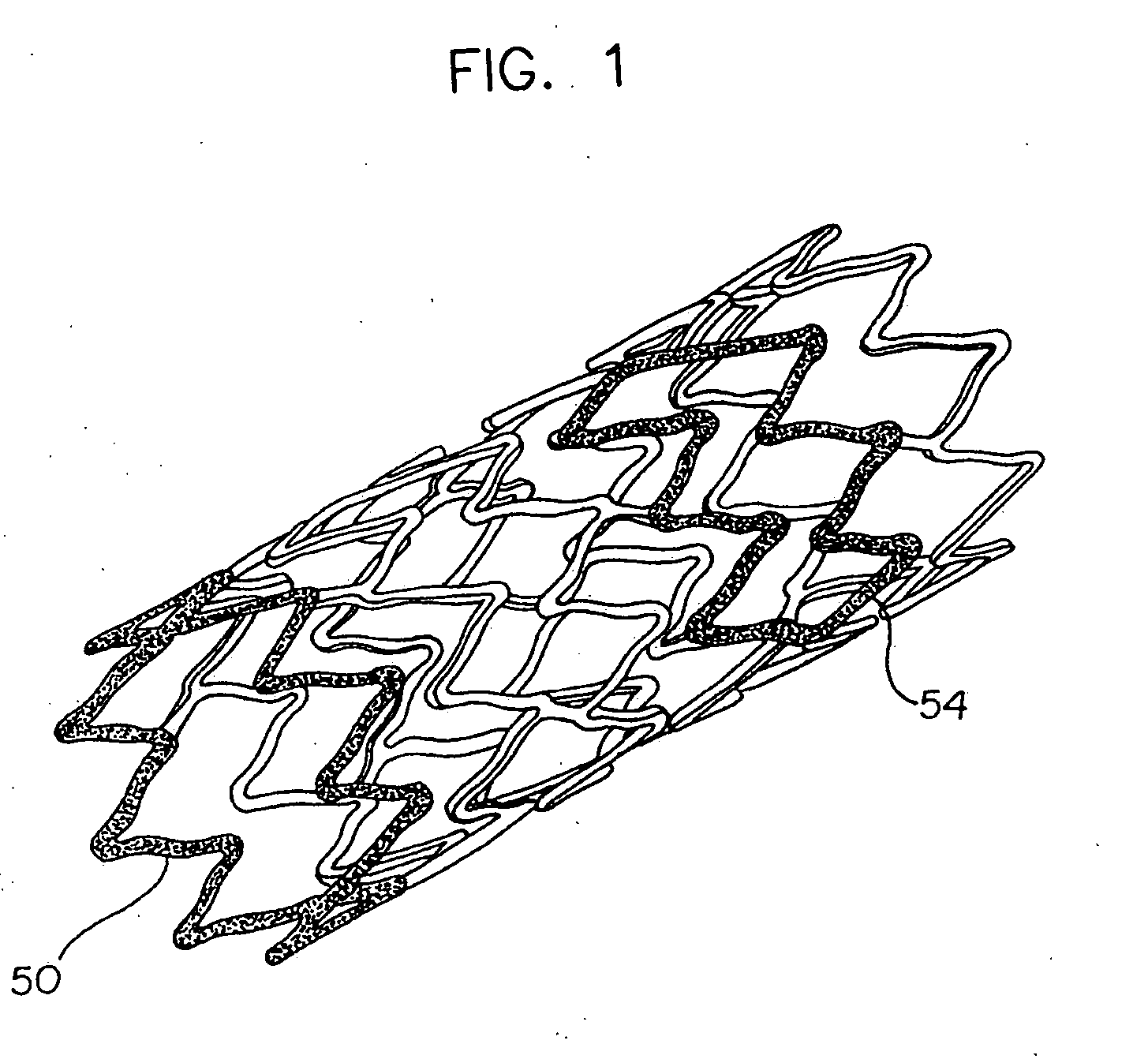
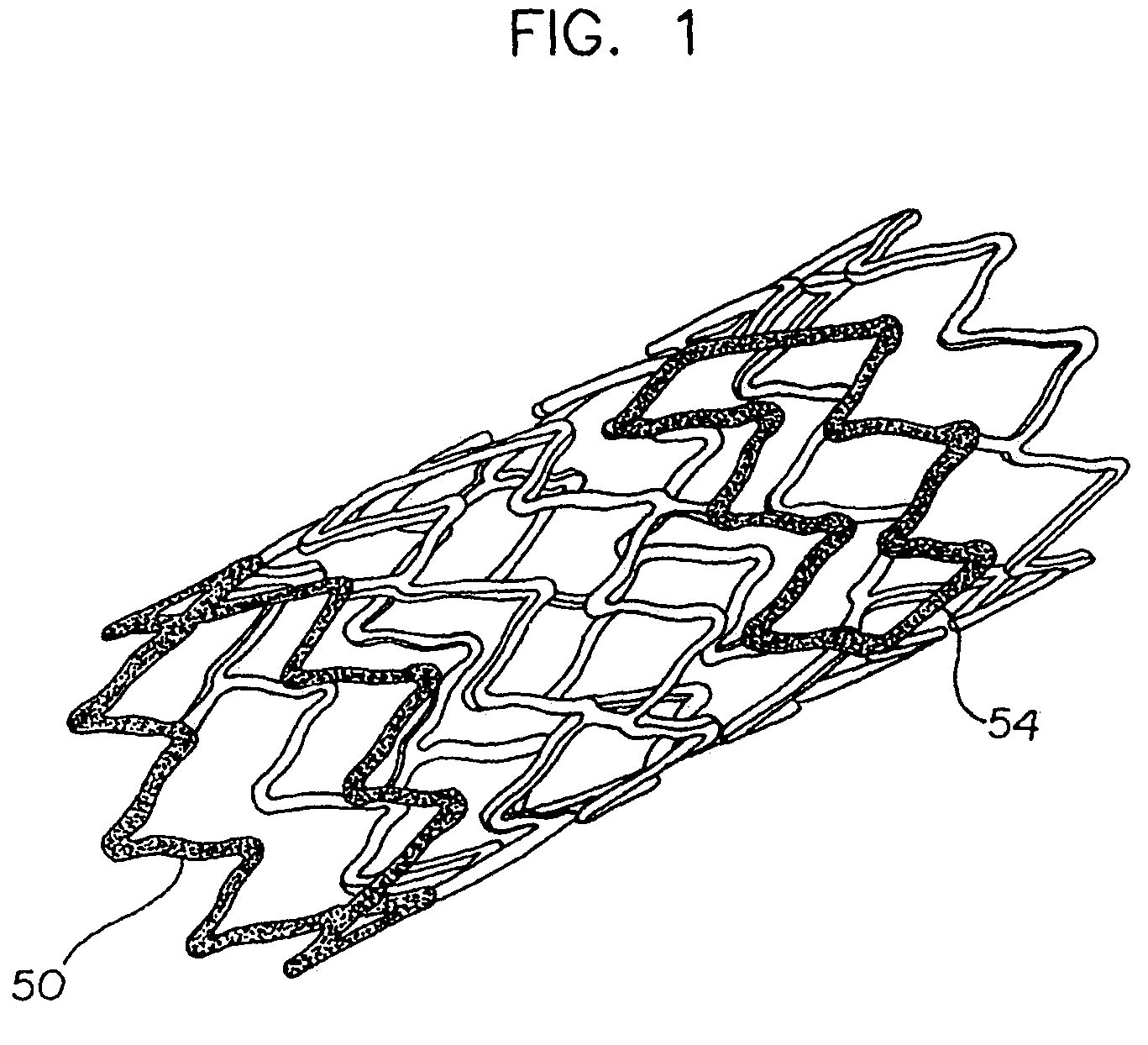
Stent with variable properties
InactiveUS7226475B2Increase flexibilityExcellent fluoroscopic property propertyStentsMedical devicesVariable thicknessMedicine
A stent having variable thickness with improved radiopacity, MRI compatibility, radial stiffness, and flexibility and to the method of making such stents.
Owner:BOSTON SCI SCIMED INC
Tank filters utilizing very low K materials, in series with lead wires or circuits of active medical devices to enhance MRI compatibility
ActiveUS7853324B2High impedanceMuch smaller and volumetrically efficientMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A TANK filter is provided for a lead wire of an active medical device (AMD). In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device. The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency to attenuate current flow through the lead wire along a range of selected frequencies. In a particularly preferred form, the TANK filter is manufactured using very low k materials of sufficient strength to handle forces applied thereto during installation and use.
Owner:WILSON GREATBATCH LTD
Metal alloy for medical devices and implants
The present invention relates to a medical device or implant made at least in part of a high strength, low modulus metal alloy comprising Niobium, Tantalum, and at least one element selected from the group consisting of Zirconium, Tungsten and Molybdenum. The medical devices according to the present invention provide superior characteristics with regard to biocompatibility, radio-opacity and MRI compatibility.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Overlapped stents for scaffolding, flexibility and MRI compatibility
A tubular insert for a vessel comprises an inner stent and an outer stent. At least a portion of the inner stent is disposed within the outer stent. The outer stent has a longitudinal axis and is constructed to be free of any closed loops which are electrically conductive and which are disposed about the longitudinal axis such that the longitudinal axis passes through the closed loop. The inner stent has a longitudinal axis and is constructed so as to be free of any closed loops which are electrically conductive and which are disposed about the longitudinal axis such that the longitudinal axis passes through the closed loop. There is a substantially electrically non-conductive connection between the inner and outer stents. Desirably, a wall surface is defined by the outer and inner stents, and there are no closed, electrically conductive loops in the wall surface of the tubular insert.
Owner:BOSTON SCI SCIMED INC
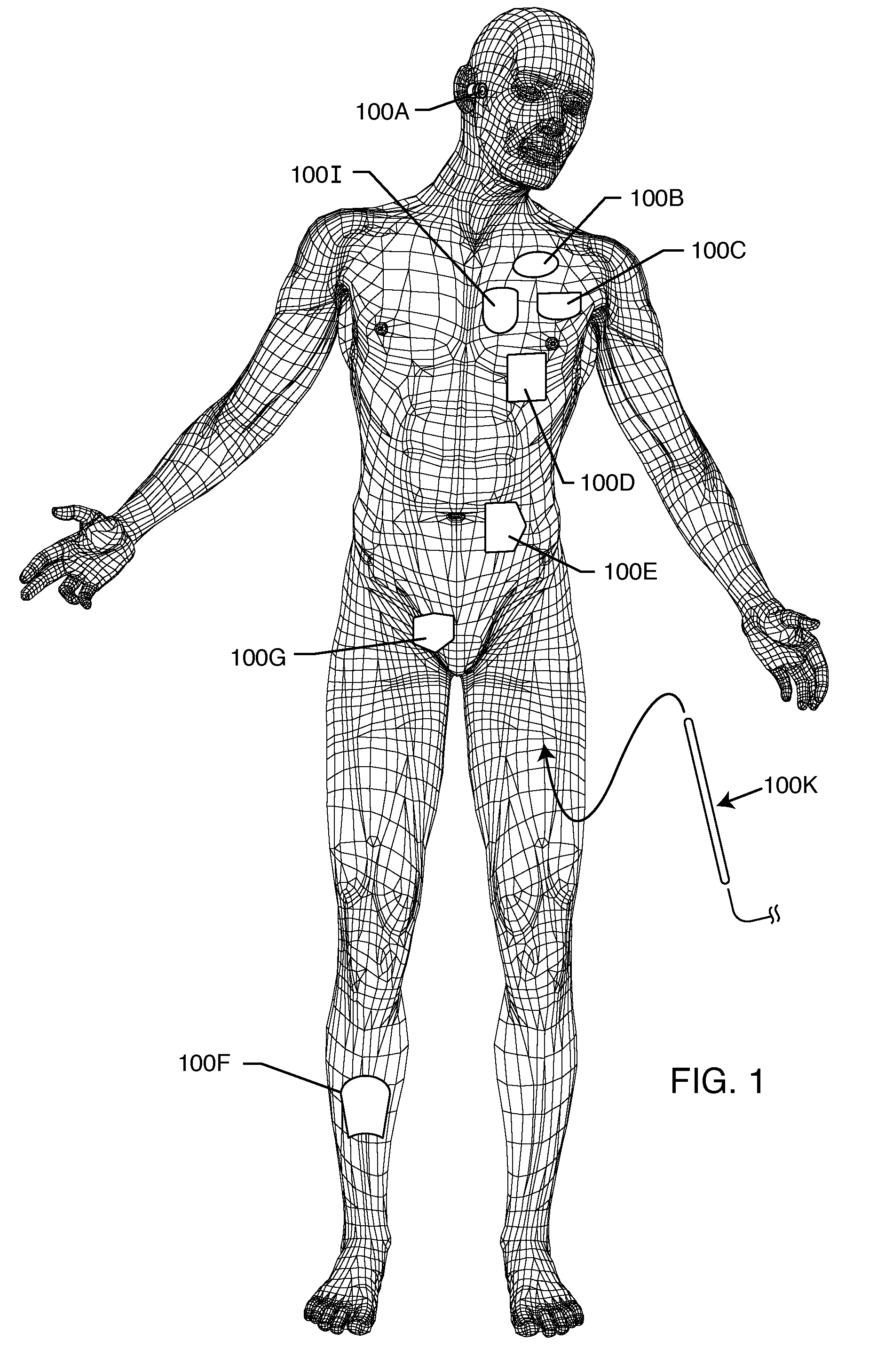
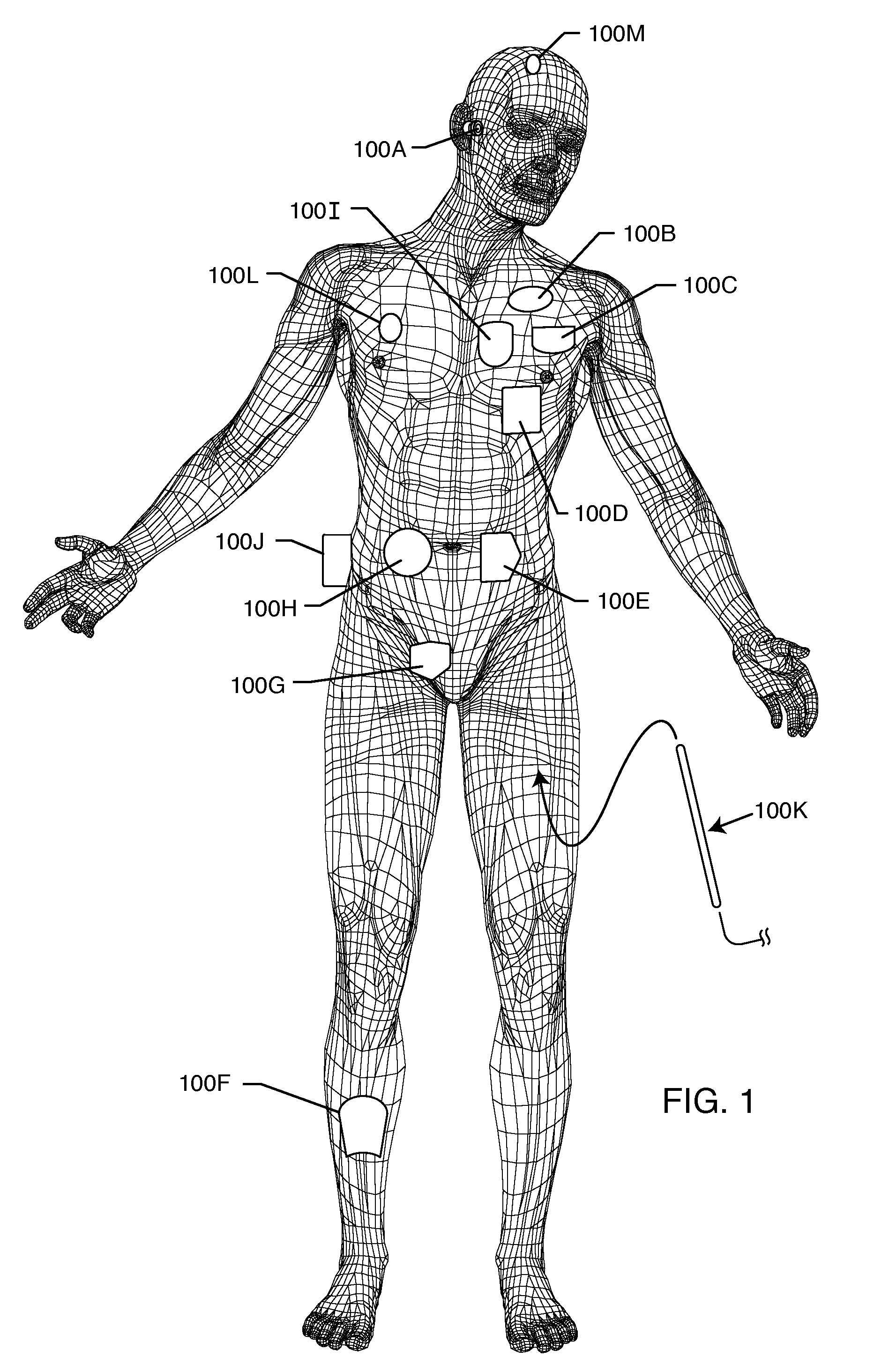
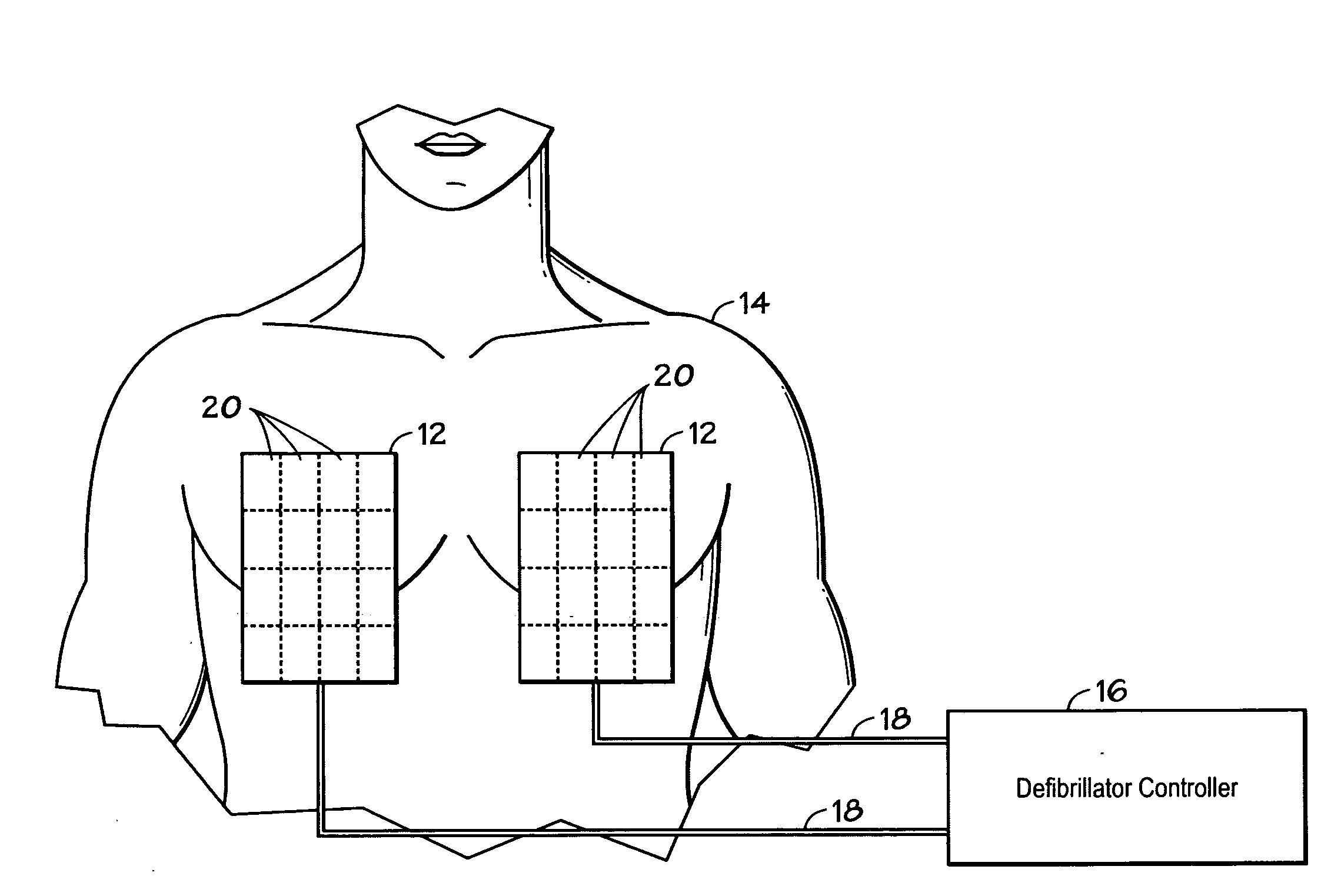
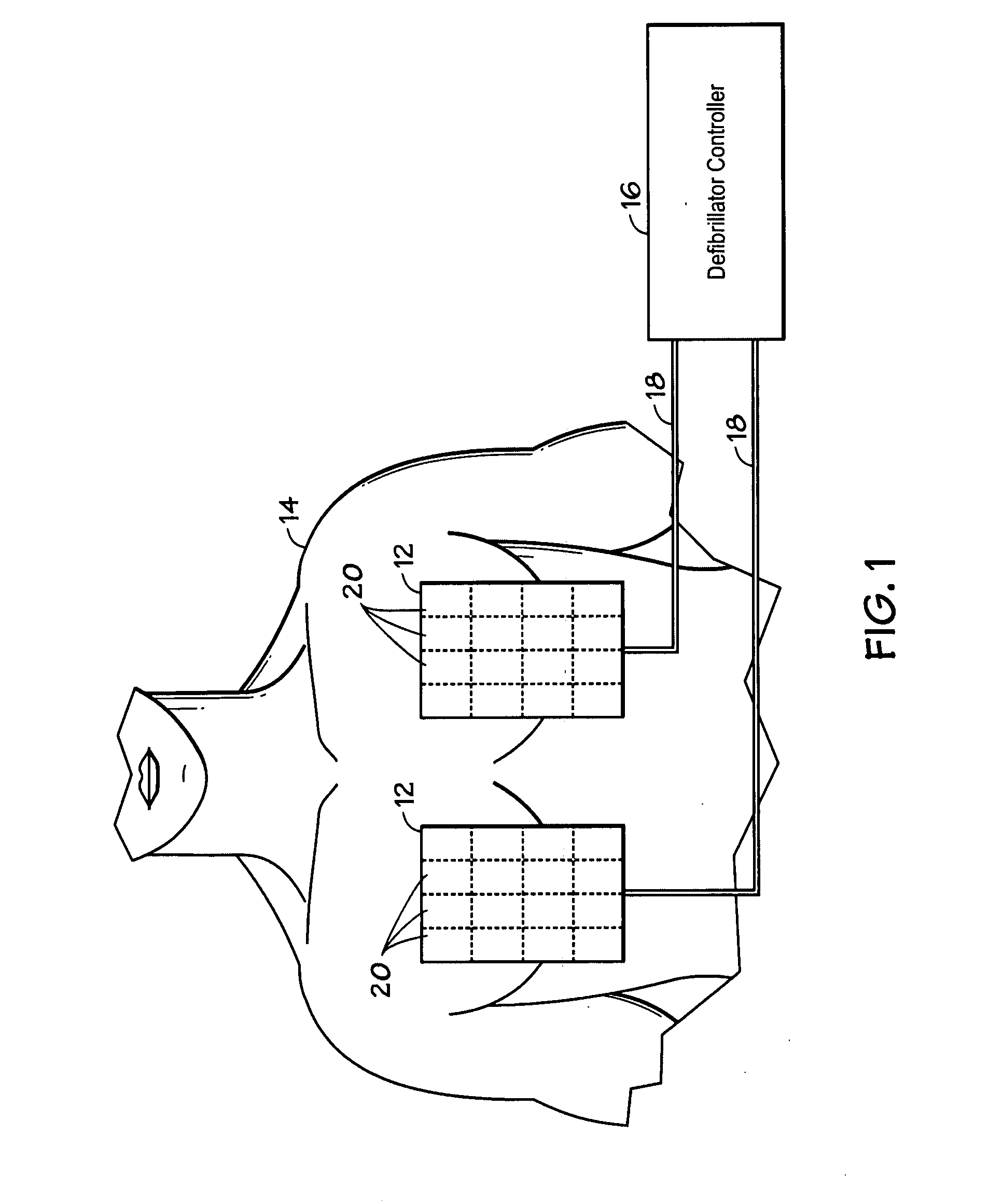
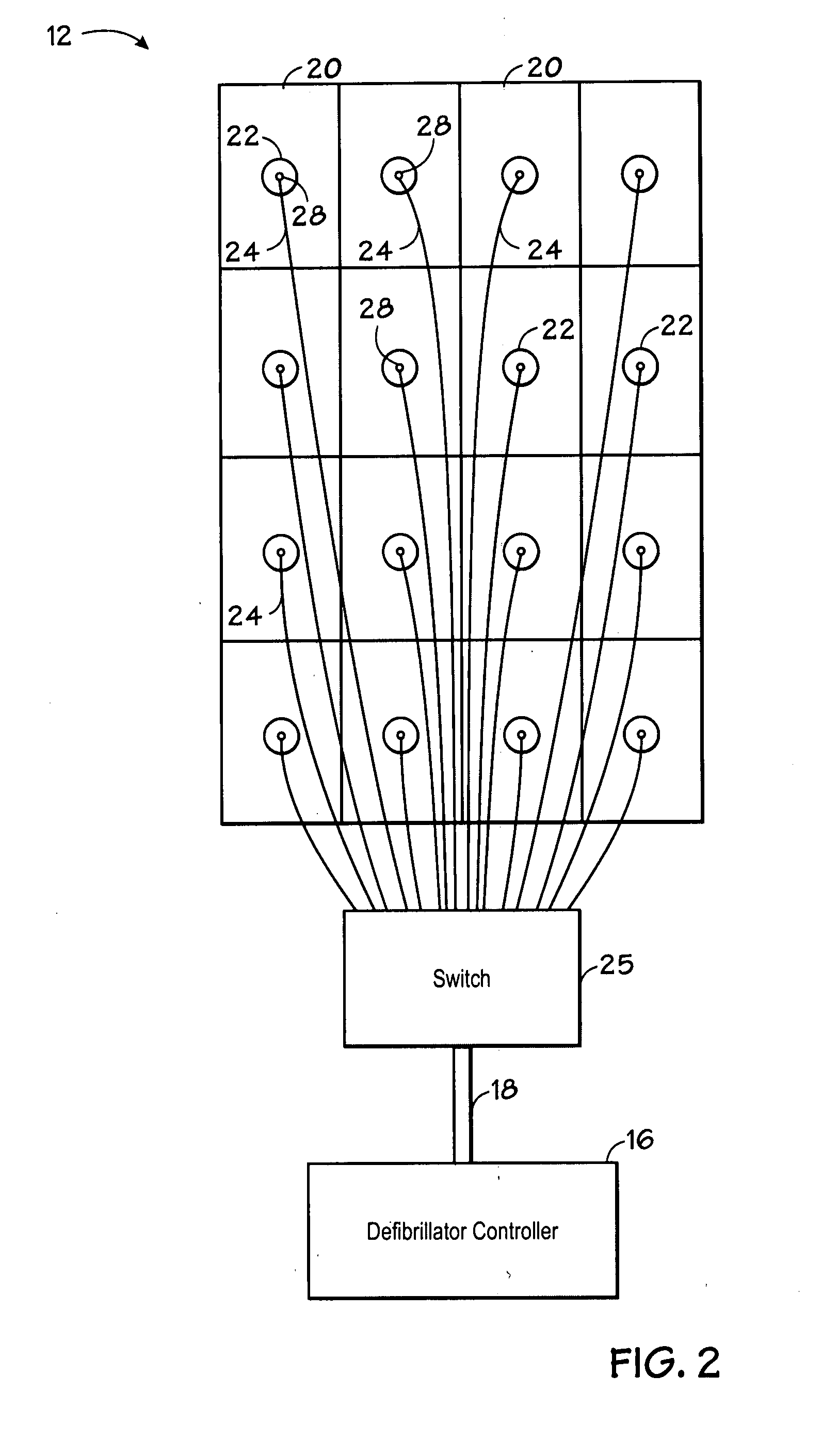
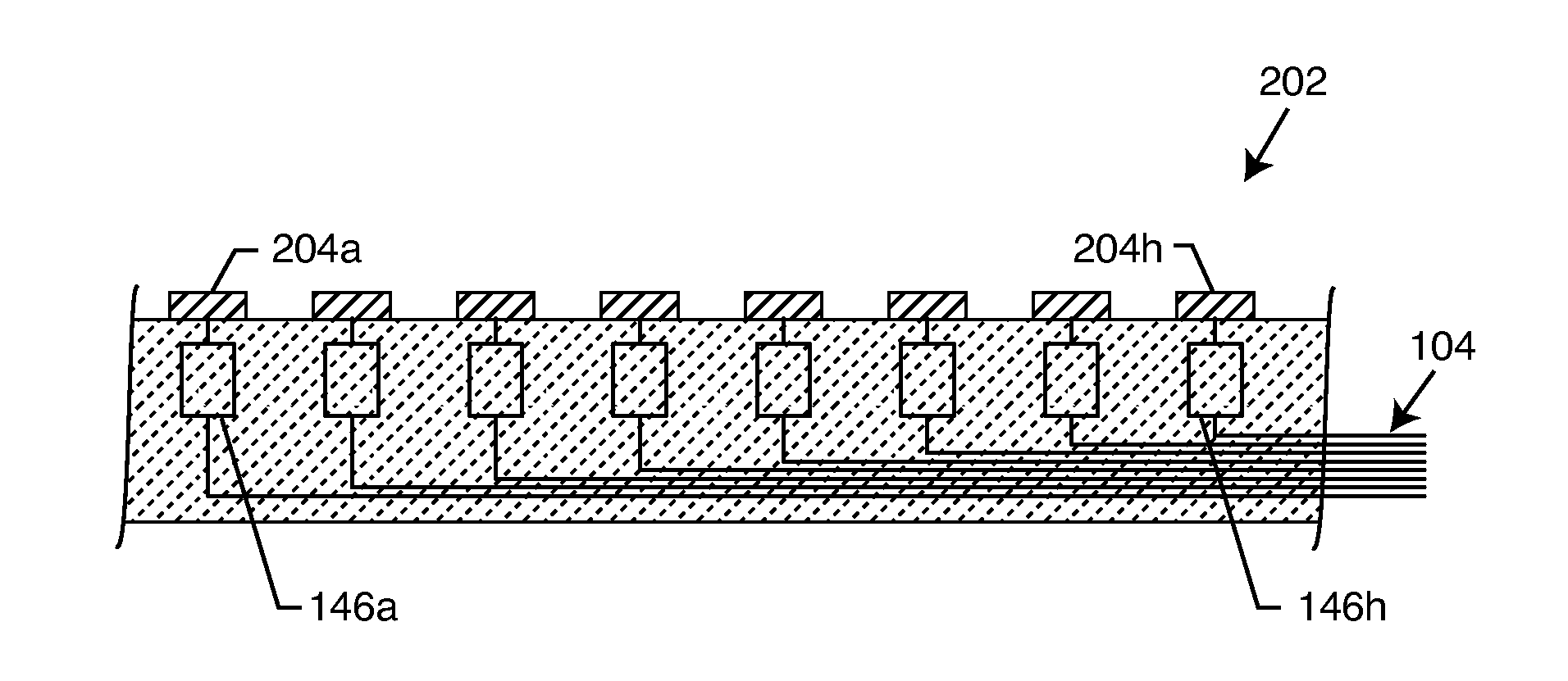
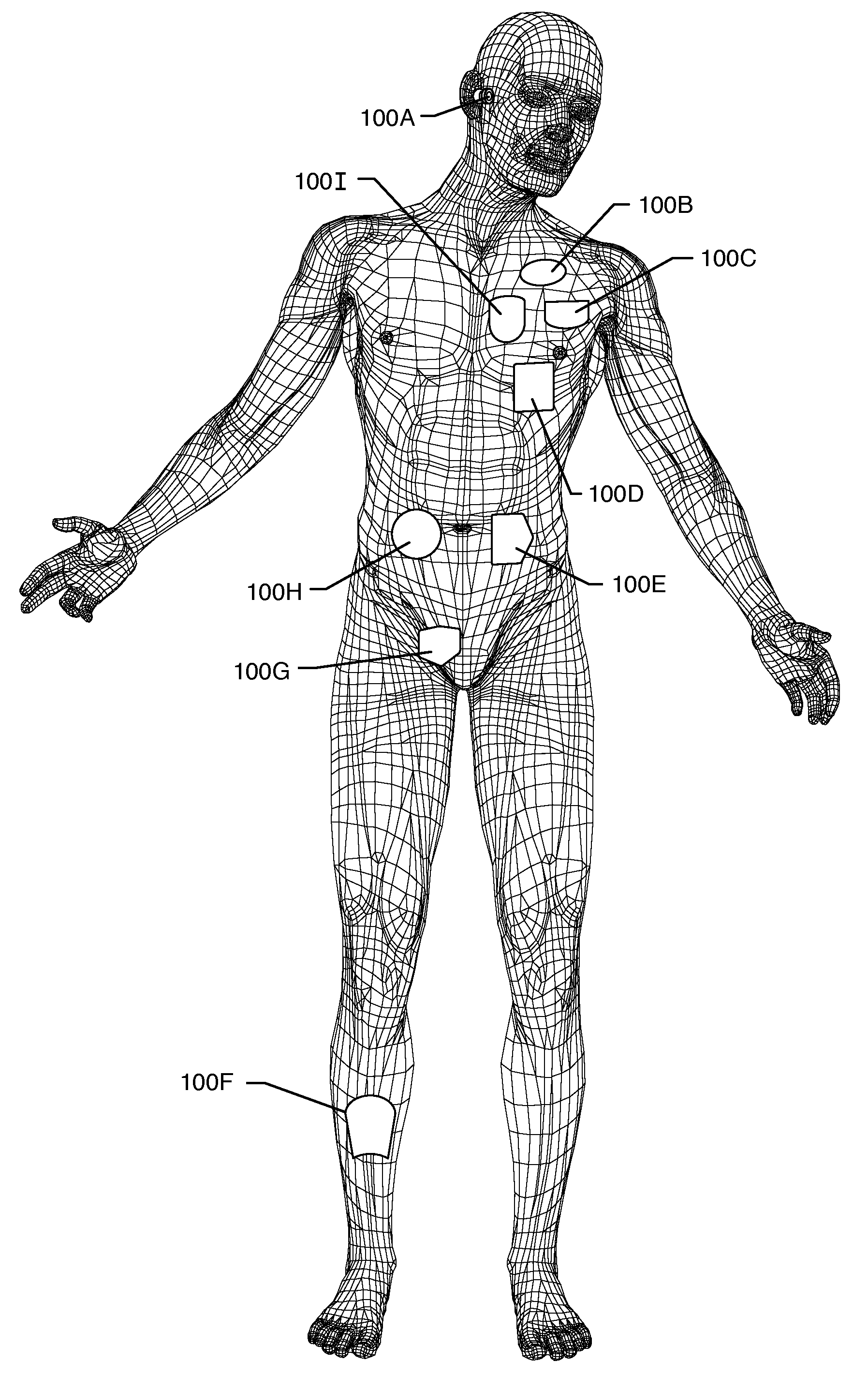
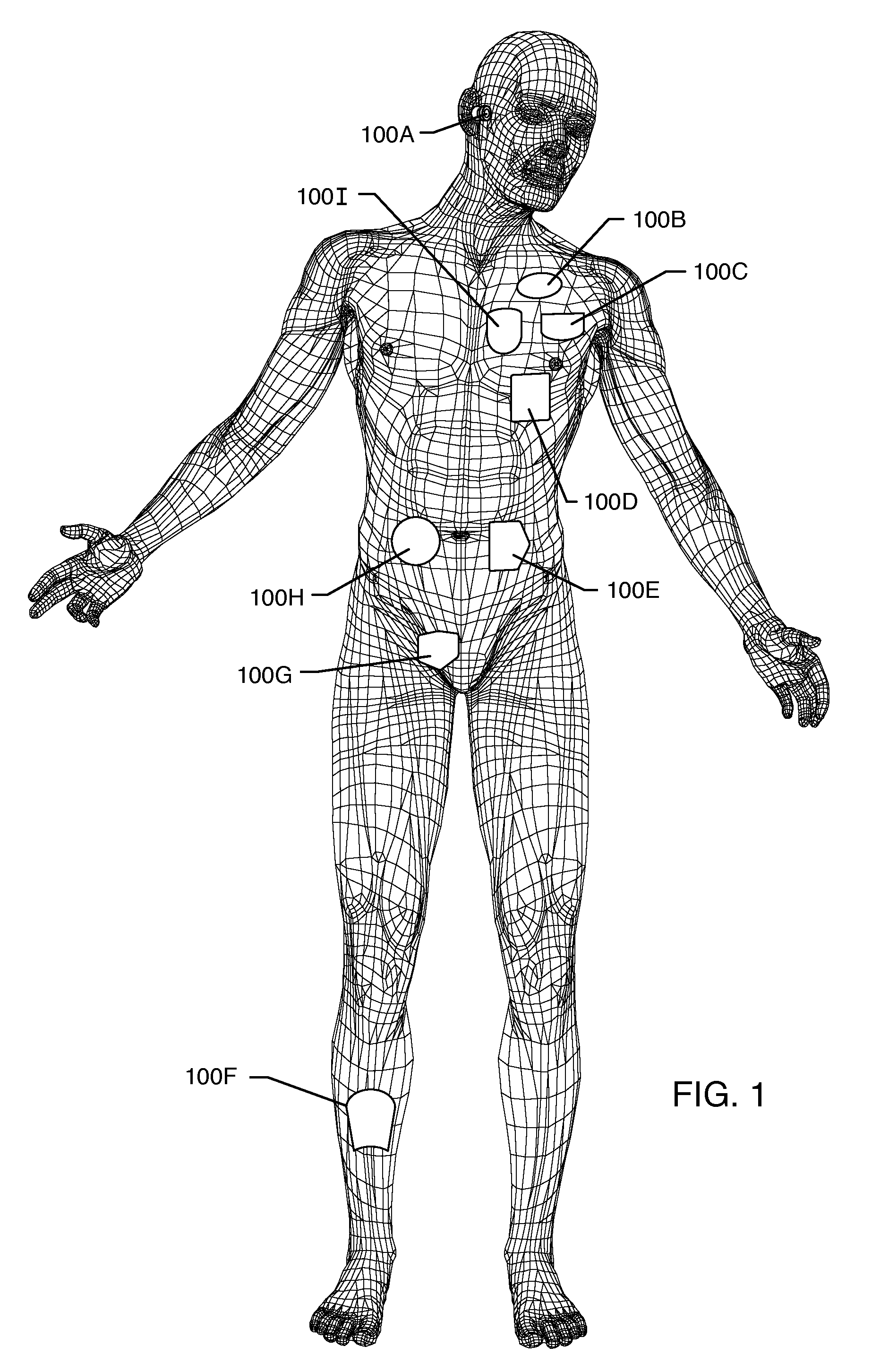
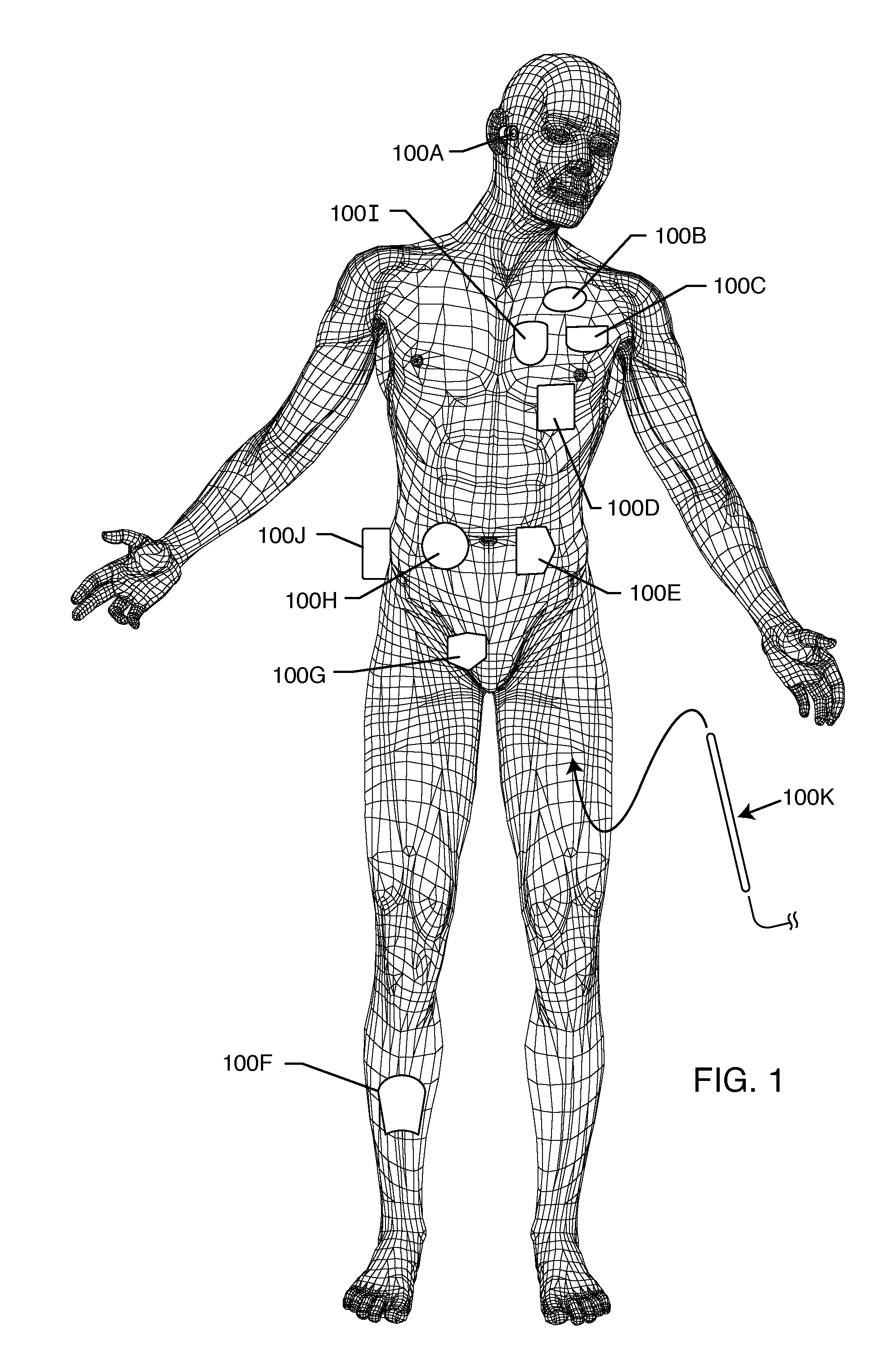
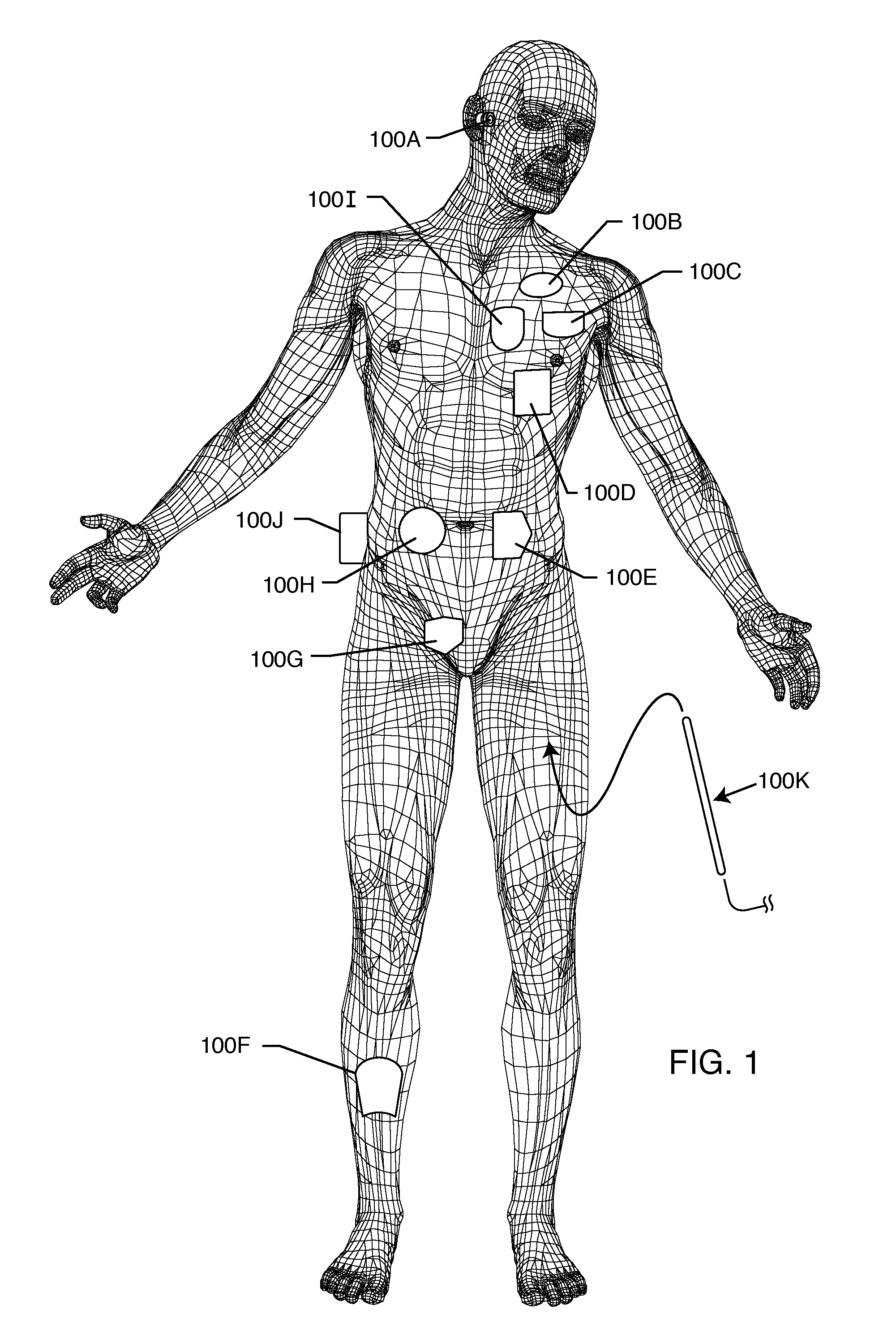
Method and system using MRI compatibility defibrillation pads
InactiveUS20080200973A1Eliminate time lagMinimizes imaging artifactInternal electrodesExternal electrodesElectricityMri compatibility
In accordance with embodiments of the present technique an electrode pad for medical use is provides. The electrode pad comprises a support layer a plurality of electrodes mounted on the support layer and electrically insulated from one another, and a plurality of leads electrically coupled to the electrodes for selectively placing the electrodes at a desired electrical potential.
Owner:GENERAL ELECTRIC CO
Overlapped stents for scaffolding, flexibility and MRI compatibility
A tubular insert for a vessel comprises an inner stent and an outer stent. At least a portion of the inner stent is disposed within the outer stent. The outer stent has a longitudinal axis and is constructed to be free of any closed loops which are electrically conductive and which are disposed about the longitudinal axis such that the longitudinal axis passes through the closed loop. The inner stent has a longitudinal axis and is constructed so as to be free of any closed loops which are electrically conductive and which are disposed about the longitudinal axis such that the longitudinal axis passes through the closed loop. There is a substantially electrically non-conductive connection between the inner and outer stents. Desirably, a wall surface is defined by the outer and inner stents, and there are no closed, electrically conductive loops in the wall surface of the tubular insert.
Owner:BOSTON SCI SCIMED INC
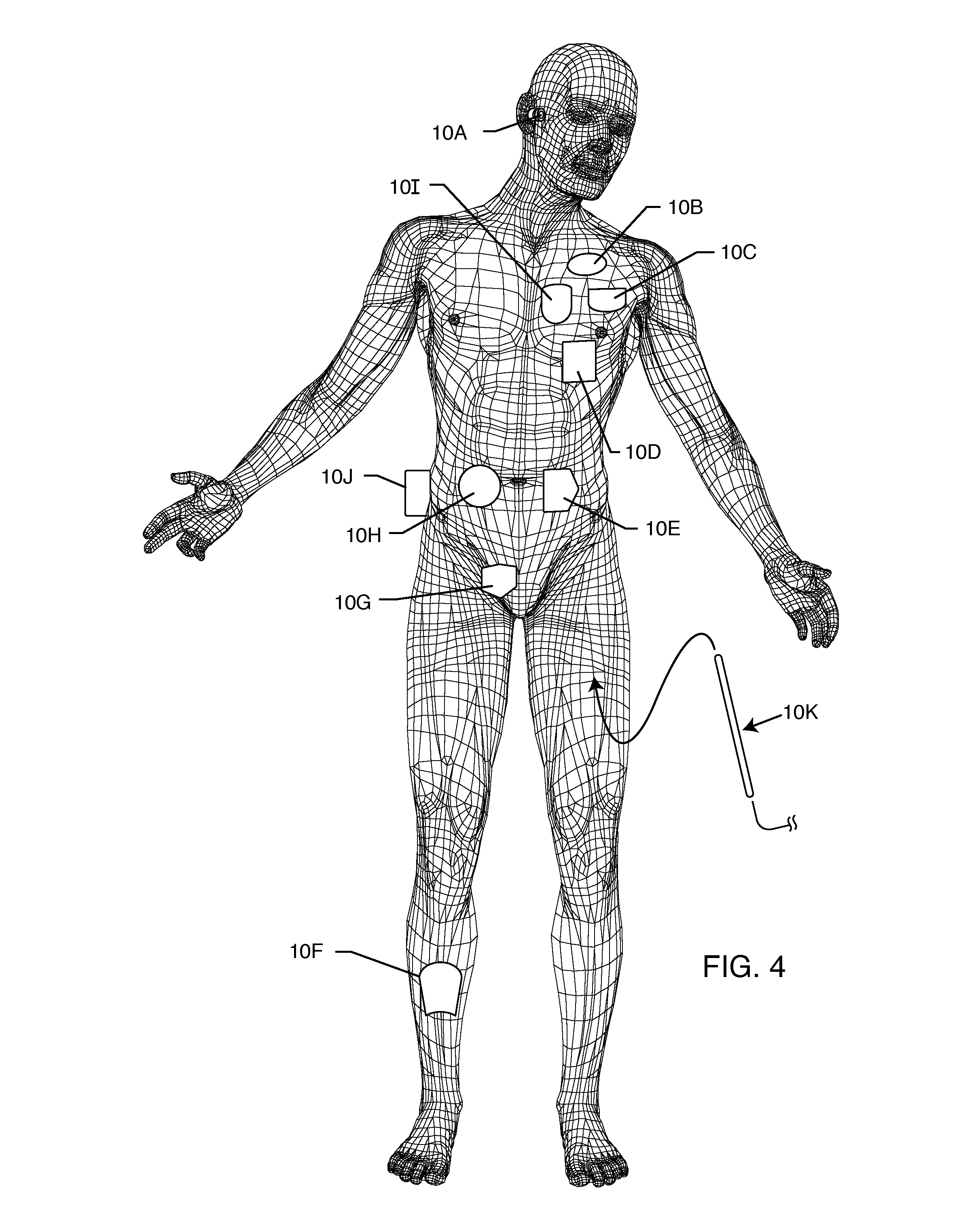
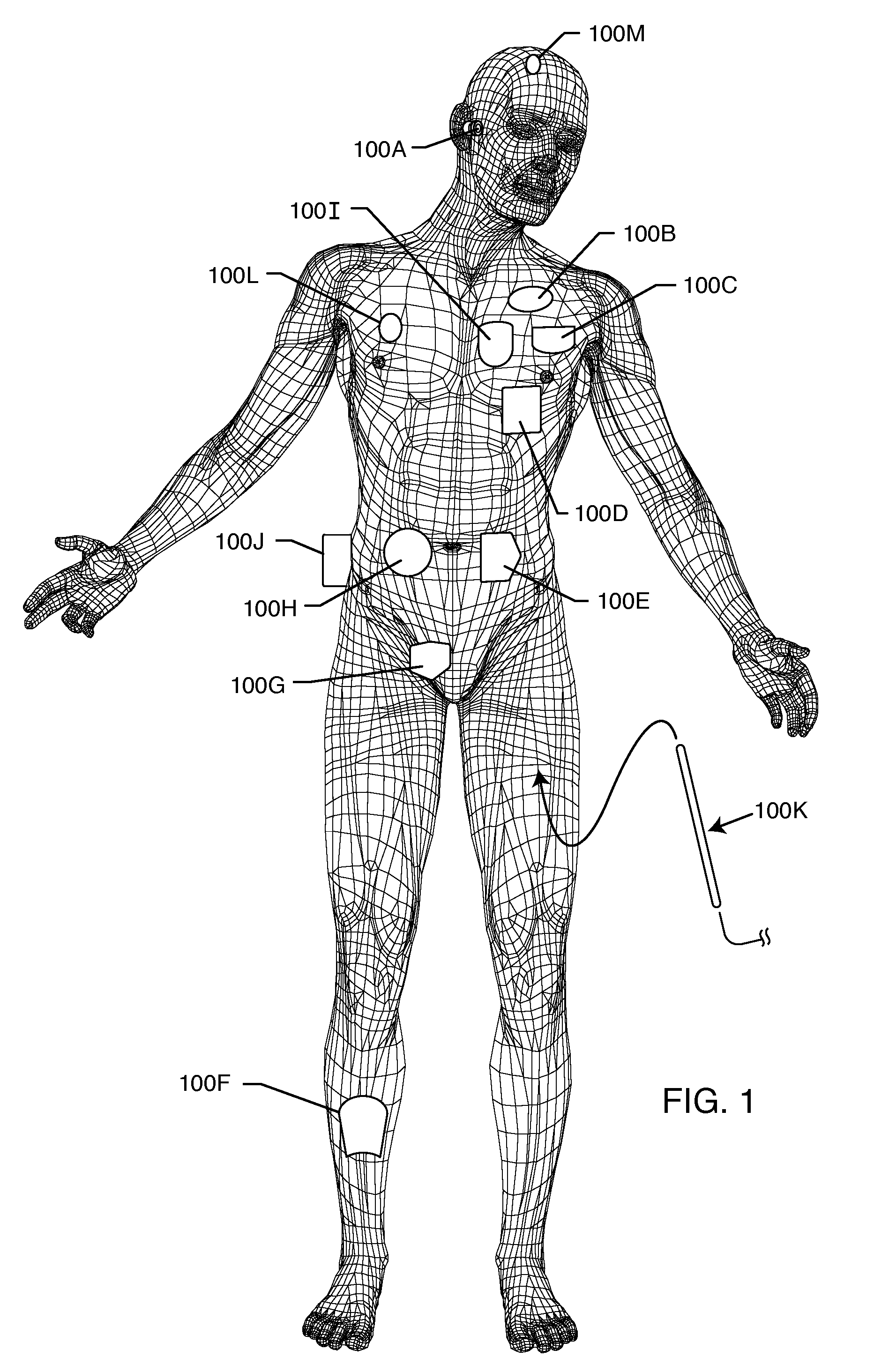
Rfid-enabled aimd programmer system for identifying MRI compatibility of implanted leads
InactiveUS20110029043A1Transvascular endocardial electrodesSurgeryTelecommunications linkGoal programming
An RFID tag is associated with an implantable lead, its sensing or therapy delivery electrode, or a patient, for identifying the MRI compatibility of the implantable lead and / or the presence of a bandstop filter and its attendant characteristics. An RFID-enabled AIMD external telemetry programmer transmits an electromagnetic signal to establish a communication link with the RFID tag.
Owner:WILSON GREATBATCH LTD
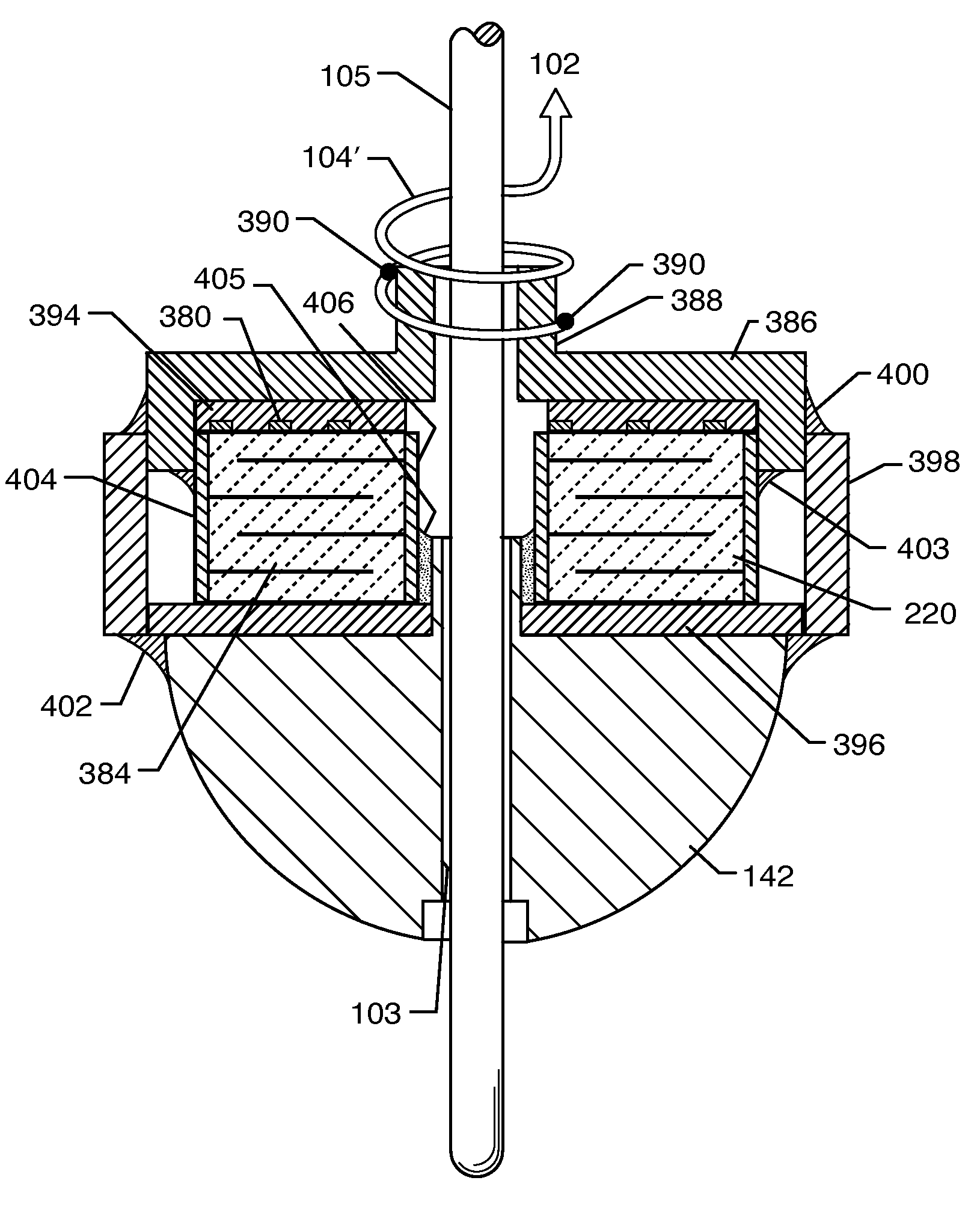
Tank filters placed in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
ActiveUS20110213233A1Fast imagingReduce sensitivityElectrocardiographyInternal electrodesCapacitanceInductor
A TANK filter is provided for a lead wire of an active medical device (AMD). The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the TANK filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Implantable lead bandstop filter employing an inductive coil with parasitic capacitance to enhance MRI compatibility of active medical devices
ActiveUS20120071956A1Fast imagingMaximized (or minimizedMultiple-port networksSpinal electrodesParasitic capacitanceLead system
A medical lead system includes at least one bandstop filter for attenuating current flow through the lead across a range of frequencies. The bandstop filter has an overall circuit Q wherein the resultant 3 dB bandwidth is at least 10 kHz. The values of capacitance and inductance of the bandstop filter are selected such that the bandstop filter is resonant at a selected center frequency or range of frequencies. Preferably, the bandstop filter has an overall circuit Q wherein the resultant 10 dB bandwidth is at least 10 kHz. Such bandstop filters are backwards compatible with known implantable deployment systems and extraction systems.
Owner:WILSON GREATBATCH LTD
Overlapped stents for scaffolding, flexibility and MRI compatibility
Owner:BOSTON SCI SCIMED INC
Tank filters placed in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
A TANK filter is provided for a lead wire of an active medical device (AMD). The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the TANK filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Tank filters placed in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS20110213232A1Fast imagingReduce sensitivityElectrocardiographyInternal electrodesCapacitanceEngineering
A TANK filter is provided for a lead wire of an active medical device (AMD). The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the TANK filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
RFID-enabled AIMD programmer system for identifying MRI compatibility of implanted leads
InactiveUS8321032B2Transvascular endocardial electrodesSurgeryTelecommunications linkCommunication link
An RFID tag is associated with an implantable lead, its sensing or therapy delivery electrode, or a patient, for identifying the MRI compatibility of the implantable lead and / or the presence of a bandstop filter and its attendant characteristics. An RFID-enabled AIMD external telemetry programmer transmits an electromagnetic signal to establish a communication link with the RFID tag.
Owner:WILSON GREATBATCH LTD
Metal alloy for medical devices and implants
InactiveUS20070221300A1Maintain good propertiesImprove uniformityStentsHeart valvesNiobiumBiocompatibility Testing
The present invention relates to a medical device or implant made at least in part of a high strength, low modulus metal alloy comprising Niobium, Tantalum, and at least one element selected from the group consisting of Zirconium, Tungsten, and Molybdenum. The medical devices according to the present invention provide superior characteristics with regard to biocompatibility, radio-opacity and MRI compatibility.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Implantable lead bandstop filter employing an inductive coil with parasitic capacitance to enhance MRI compatibility of active medical devices
ActiveUS8903505B2Maximized (or minimizedMinimize resistance lossSpinal electrodesMultiple-port networksParasitic capacitanceLead system
A medical lead system includes at least one bandstop filter for attenuating current flow through the lead across a range of frequencies. The bandstop filter has an overall circuit Q wherein the resultant 3 dB bandwidth is at least 10 kHz. The values of capacitance and inductance of the bandstop filter are selected such that the bandstop filter is resonant at a selected center frequency or range of frequencies. Preferably, the bandstop filter has an overall circuit Q wherein the resultant 10 dB bandwidth is at least 10 kHz. Such bandstop filters are backwards compatible with known implantable deployment systems and extraction systems.
Owner:WILSON GREATBATCH LTD
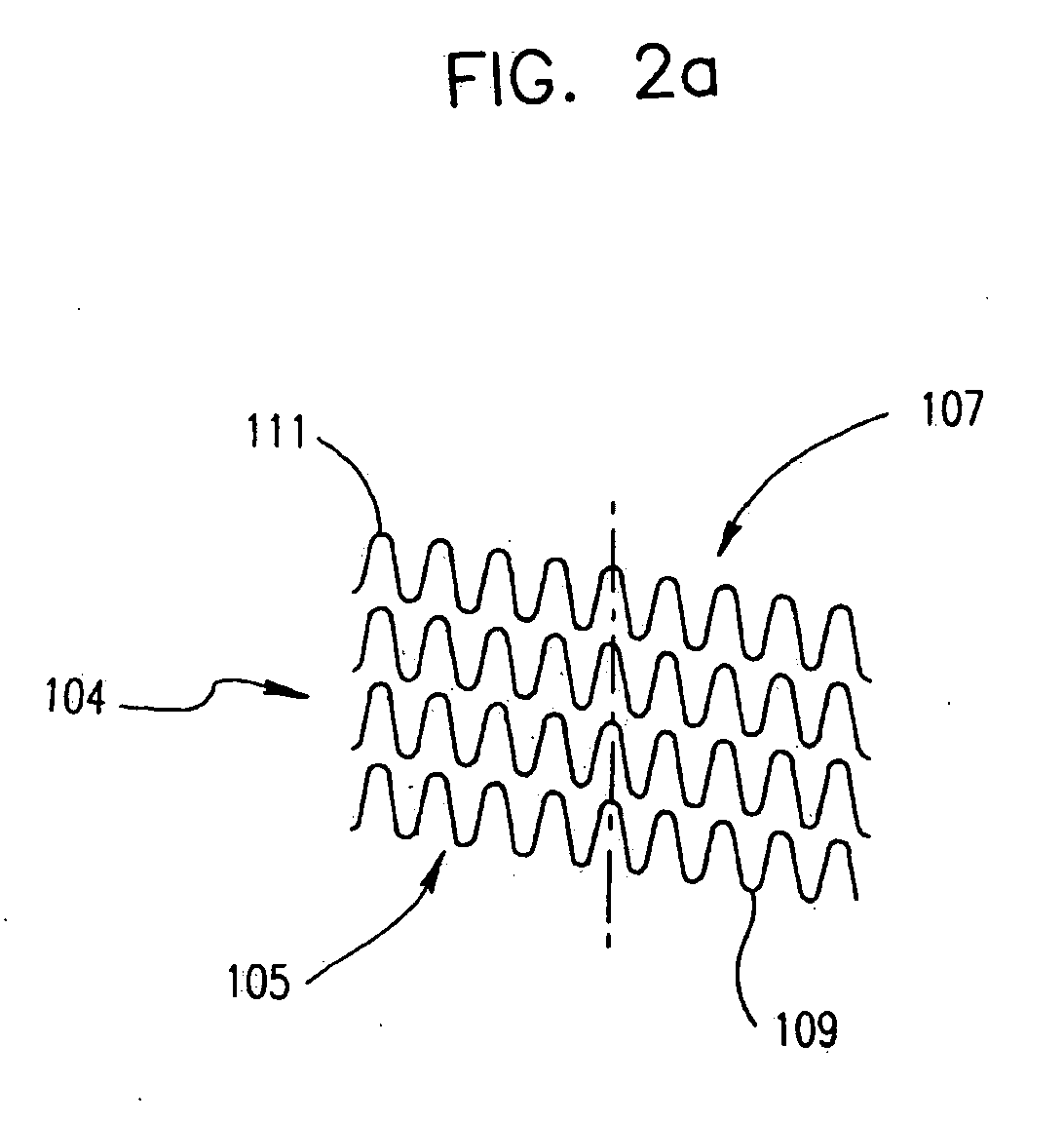
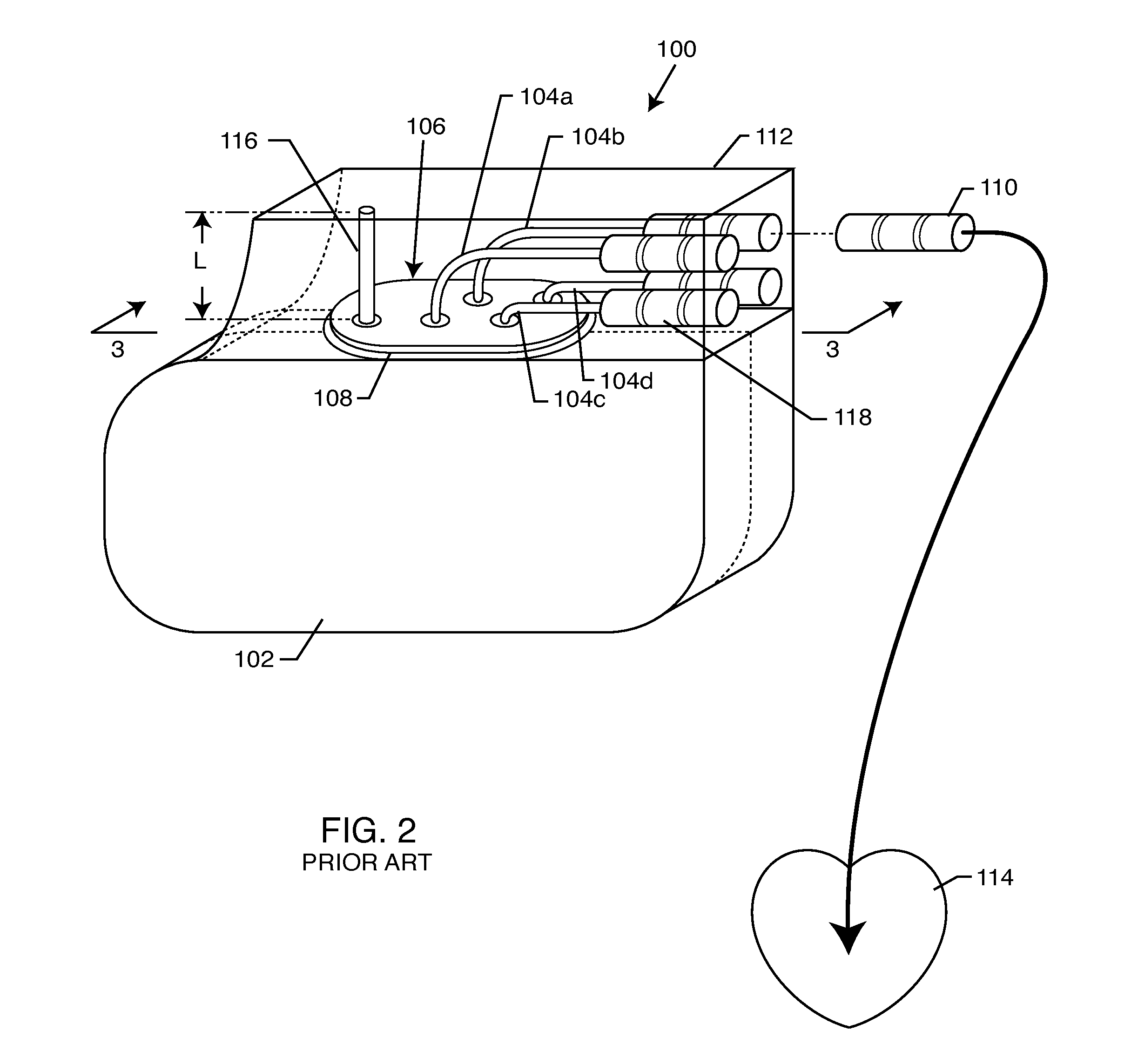
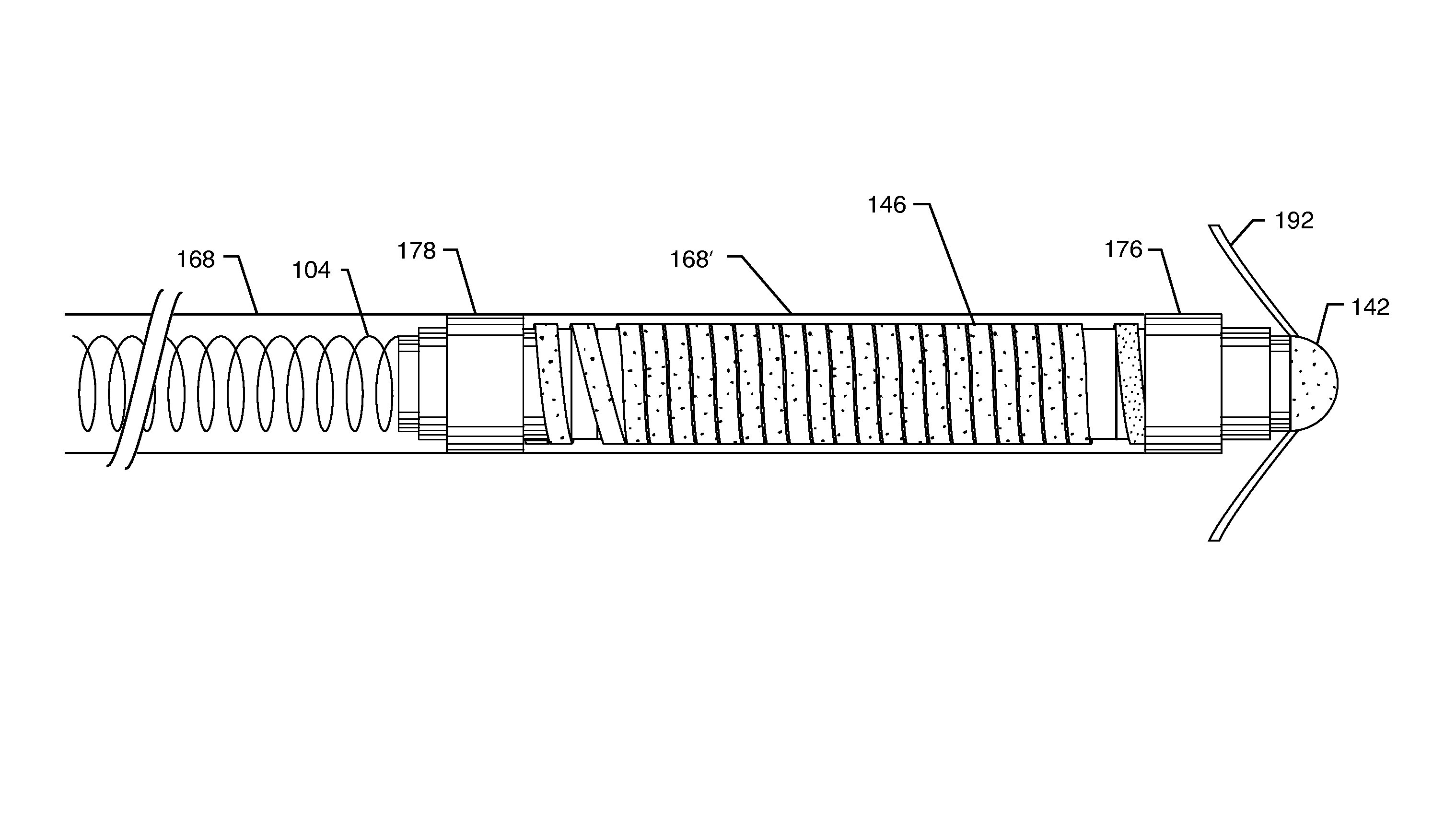
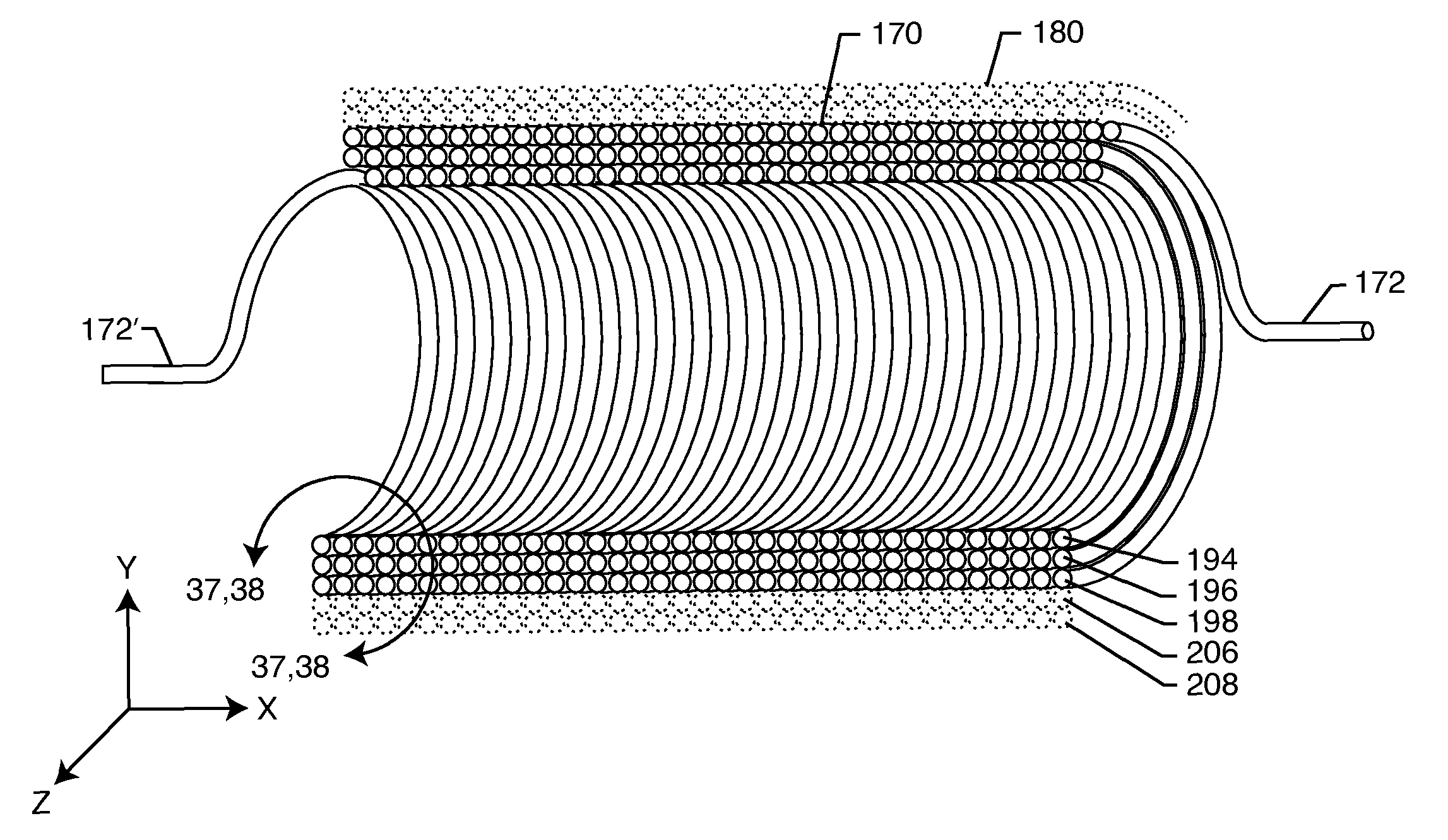
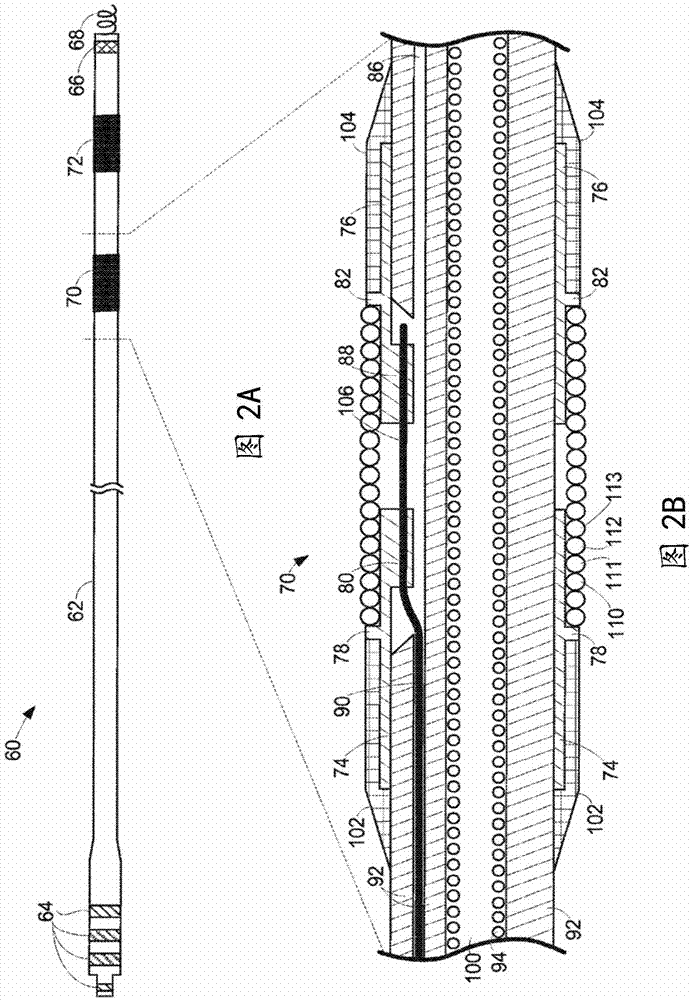
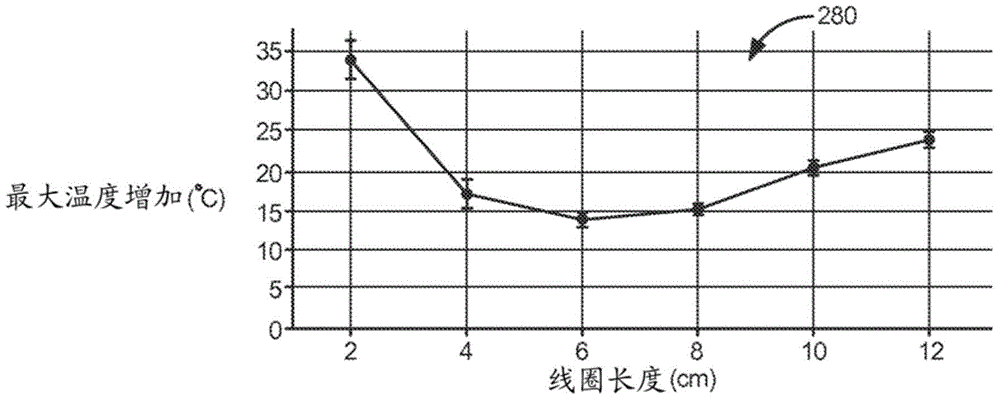
Self-resonant inductor wound portion of an implantable lead for enhanced MRI compatibility of active implantable medical devices
An implantable lead includes a lead conductor having a length extending from a proximal end to a distal end. A self-resonant inductor is connected in series along a portion of the length of the lead conductor. The self-resonant inductor includes a single length of conductive material including a dielectric coating substantially surrounding the single length of conductive material. The self-resonant inductor includes a first coiled or spiral conductor disposed along an inductor section spanning in a first direction from a first location to a second location. A second coiled or spiral conductor is disposed along the inductor section spanning in a second direction from the second location to the first location, where the second direction is opposite the first direction. A third coiled or spiral conductor is disposed along the inductor section spanning in the first direction from the first location to the second location.
Owner:WILSON GREATBATCH LTD
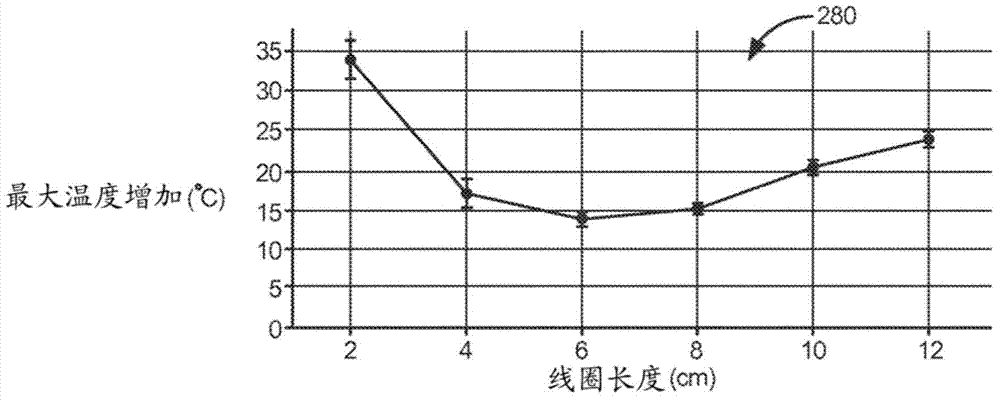
Mri compatible lead coil
Various embodiments concern leads having low peak MRI heating for improved MRI compatibility. Various leads include a lead body having at least one lumen, a proximal end configured to interface with an implantable medical device, and a distal end. Such leads can further include a conductor extending along at least a portion of the lead body within the at least one lumen and a defibrillation coil extending along an exterior portion of the lead body and in electrical connection with the conductor, wherein at least a section of the defibrillation coil is under longitudinal compression. The longitudinal compression can lower peak MRI heating along the defibrillation coil. The longitudinal compression may maintain circumferential contact between adjacent turns of the section of the defibrillation coil.
Owner:CARDIAC PACEMAKERS INC
mri compatible lead coil
Various embodiments relate to leads with low peak MRI heating for improved MRI compatibility. Various leads include: a lead body having at least one lumen; a proximal end configured to interface with an implantable medical device; and a distal end. Such a lead may further comprise: a conductor extending along at least a portion of the lead body within the at least one lumen and a defibrillation coil extending along an outer portion of the lead body and electrically connected to the conductor, wherein the defibrillation coil At least one fragment of is under vertical compression. Longitudinal compression can reduce peak MRI heating of defibrillation coils. The longitudinal compression can maintain circumferential contact between adjacent turns of the defibrillation coil segments.
Owner:CARDIAC PACEMAKERS INC
MRI compatible full-automatic mammary gland focus positioning biopsy robot system
PendingCN112754618AHas full MRI compatibilityReal-time MRI image navigationSurgical needlesSurgical navigation systemsPuncture BiopsyMachine
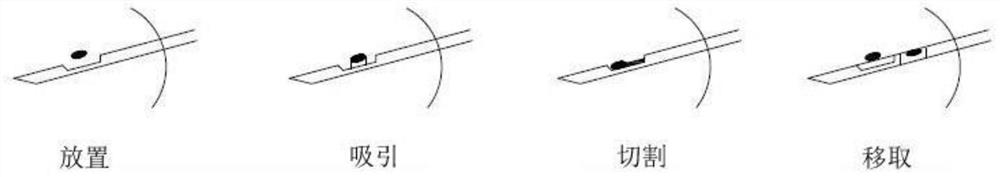
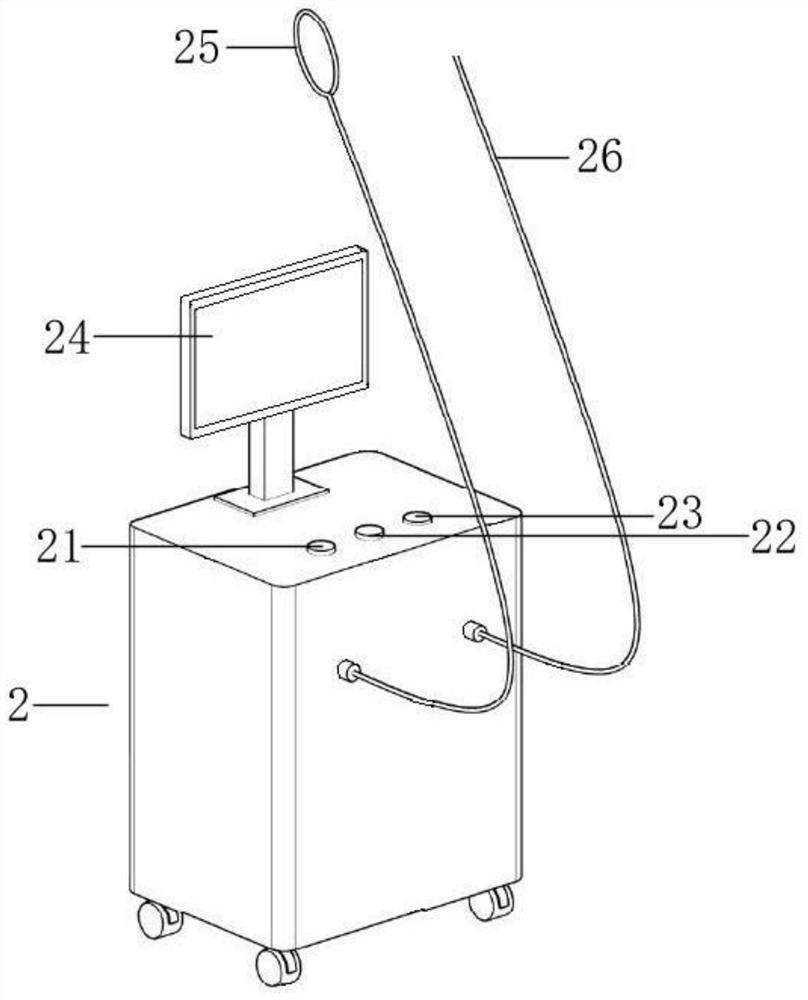
The invention relates to an MRI compatible full-automatic mammary gland focus positioning biopsy robot system. The MRI compatible full-automatic mammary gland focus positioning biopsy robot system comprises a vacuum suction biopsy instrument and a six-degree-of-freedom positioning robot system, wherein the vacuum suction biopsy instrument comprises a puncture biopsy needle and a power system host; the six-degree-of-freedom positioning robot system comprises a machine body structure and a driving system; the power system host comprises a host body; and the machine body structure comprises a positioning structure, an orientation structure and a puncture structure; the positioning structure has three degrees of freedom, the orientation structure has two degrees of freedom, and the puncture structure has one degree of freedom. The MRI compatible full-automatic mammary gland focus positioning biopsy robot system has the advantages that a full-automatic mammary gland puncture positioning robot has complete MRI compatibility, and real-time MRI image navigation of mammary gland focus sampling and automatic setting of one-time needle insertion and multiple sampling can be achieved under the assistance of a mammary gland vacuum suction rotary cutting biopsy system.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com