Coating compositions for bioactive agents
a bioactive agent and coating composition technology, applied in the field of coating compositions for bioactive agents, can solve the problems of ischemic injury, stroke or myocardial infarction, sudden closure of the vessel, and numerous physiological complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
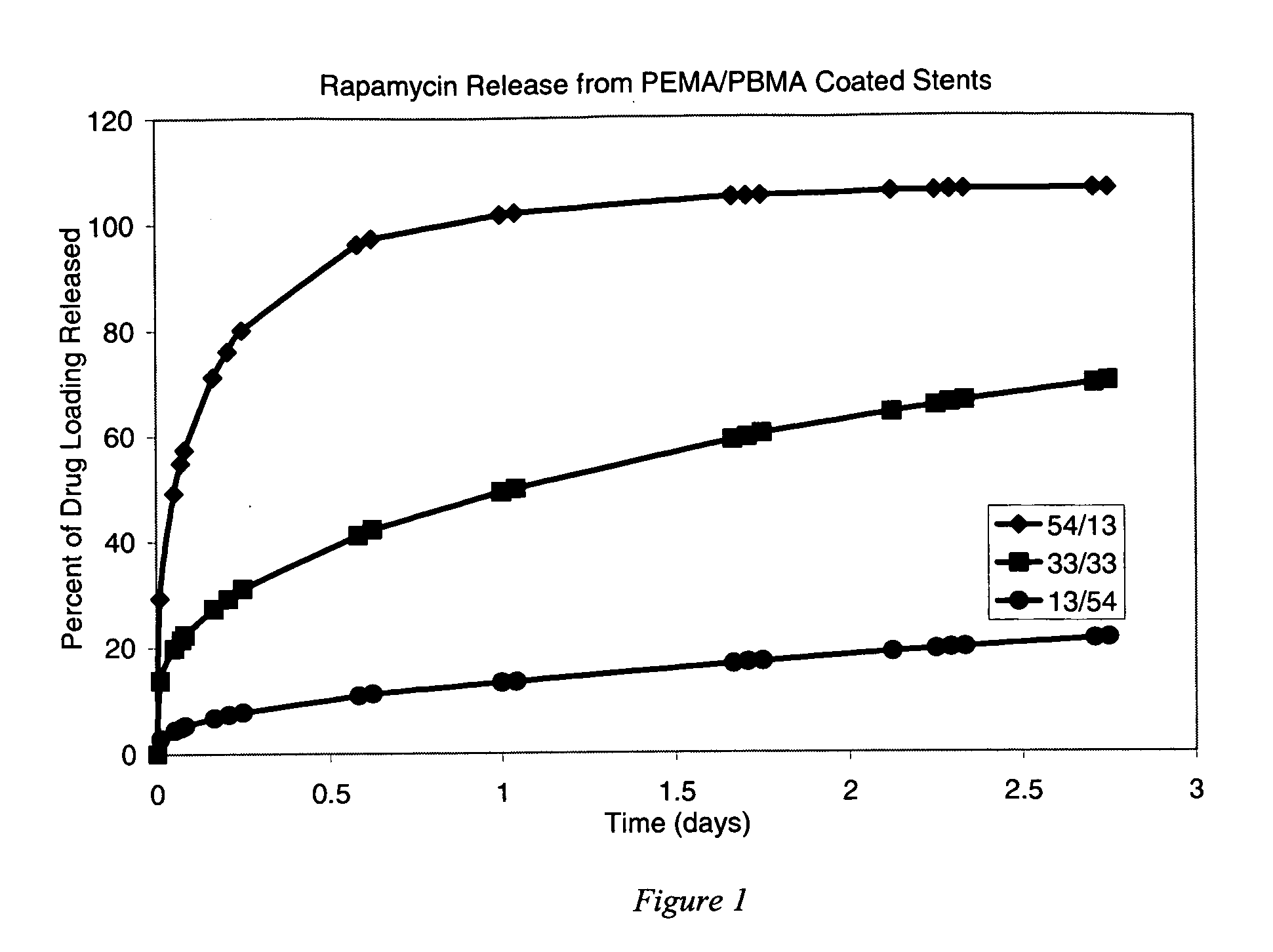
Release of Rapamycin from Poly(ethylene-co-methyl acrylate) and Poly(butyl methacrylate)
[0124] Three solutions were prepared for coating the stents. All three solutions included mixtures of poly(ethylene-co-methyl acrylate) (“PEMA”, available from Focus Chemical Corp. Portsmouth, N.H., containing 28% (wt) methyl acrylate), poly(butyl methacrylate) (“PBMA”, available from Sigma-Aldrich Fine Chemicals as Product No. 18,152-8, having a weight average molecular weight (Mw) of about 337 kilodaltons), and rapamycin (“RAPA”, available from LC Laboratories, Woburn, Mass.) dissolved in tetrahydrofuran (THF) to form a homogeneous solution. The stents were not given a primer pre-treatment.
[0125] The solutions were prepared to include the following ingredients at the stated weights per milliliter of THF: [0126] 1) 16 mg / ml PEMA / 4 mg / ml PBMA / 10 mg / ml RAPA [0127] 2) 10 mg / ml PEMA / 10 mg / ml PBMA / 10 mg / ml RAPA [0128] 3) 4 mg / ml PEMA / 16 mg / ml PBMA / 10 mg / ml RAPA
[0129] Using the Sample Preparation P...
example 2
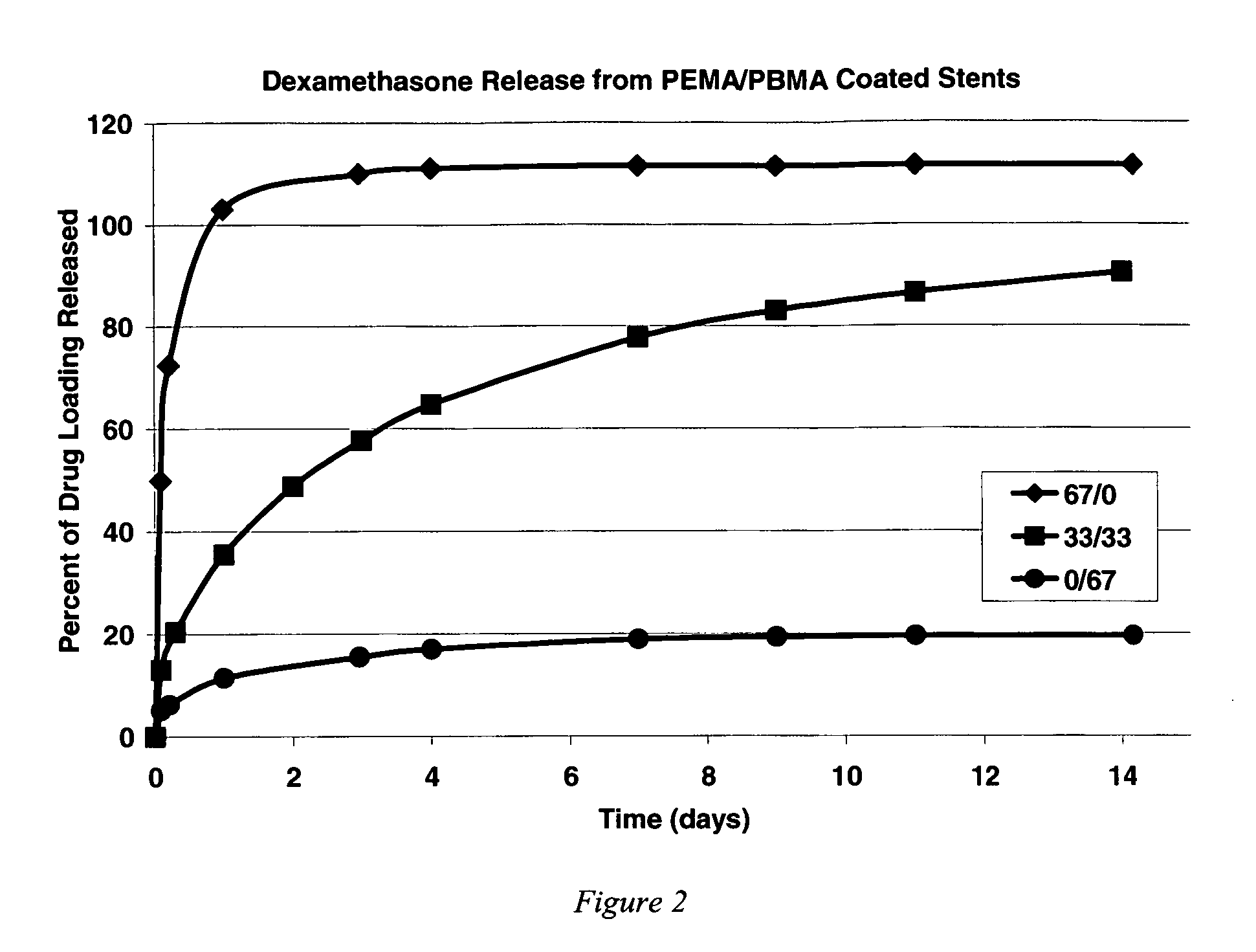
Release of Dexamethasone from Poly(ethylene-co-methyl acrylate) and Poly(butyl methacrylate)
[0131] Three solutions were prepared for coating the stents. All three solutions included mixtures of poly(ethylene-co-methyl acrylate) (“PEMA”), poly(butyl methacrylate) “PBMA”, and dexamethasone (“DEXA”, available as Product No. 86,187-1 from Sigma Aldrich Fine Chemicals) dissolved in THF to form a homogeneous solution. The stents were not given a primer pre-treatment. The solutions were prepared to include the following ingredients at the stated weights per milliliter of THF: [0132] 1) 20 mg / ml PEMA / 0 mg / ml PBMA / 10 mg / ml DEXA [0133] 2) 10 mg / ml PEMA / 10 mg / ml PBMA / 10 mg / ml DEXA [0134] 3) 0 mg / ml PEMA / 20 mg / ml PBMA / 10 mg / ml DEXA
[0135] Using the Sample Preparation Procedure, two stents were spray coated using each solution. After solvent removal via ambient evaporation, the drug elution for each coated stent was monitored using the Dexamethasone Release Assay Procedure.
[0136] Results, prov...
example 3
Surface Characterization of Coated Stents after Crimping and Expansion
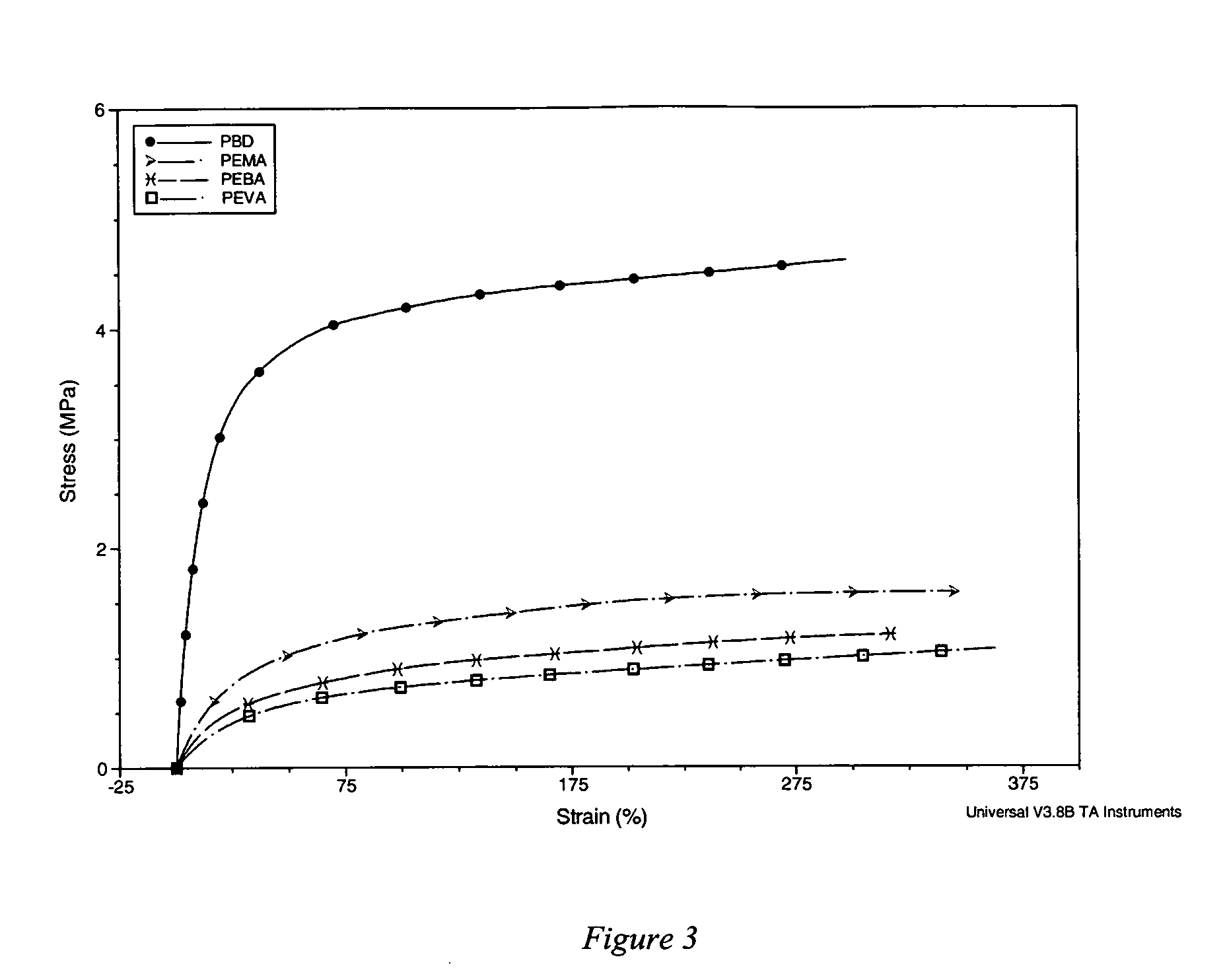
[0137] Using the Sample Preparation Procedure, stents were sprayed with a coating of second polymer / poly(butyl methacrylate)(“PBMA”) / rapamycin(“RAPA”), mixed at a weight ratio of 33 / 33 / 33 at 10 mg / ml each of THF. The first polymer was poly(ethylene-co-methyl acrylate) (“PEMA”, available from Focus Chemical Corp. Portsmouth, N.H., containing 28% (wt) methyl acrylate). The second polymer used was PBMA from Sigma-Aldrich Fine Chemicals as Product No. 18,152-8, having a weight average molecular weight (Mw) of about 337 kilodaltons. Stents were either used as received (i.e., uncoated metal), were pre-treated with a silane / Parylene™ primer using the primer procedure described in the Sample Preparation Procedure, were not pre-treated with primer but were given a subsequent PBMA topcoat using the spraying process described in the Sample Preparation Procedure, or were given both a silane / Parylene™ pre-treatment primer and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com