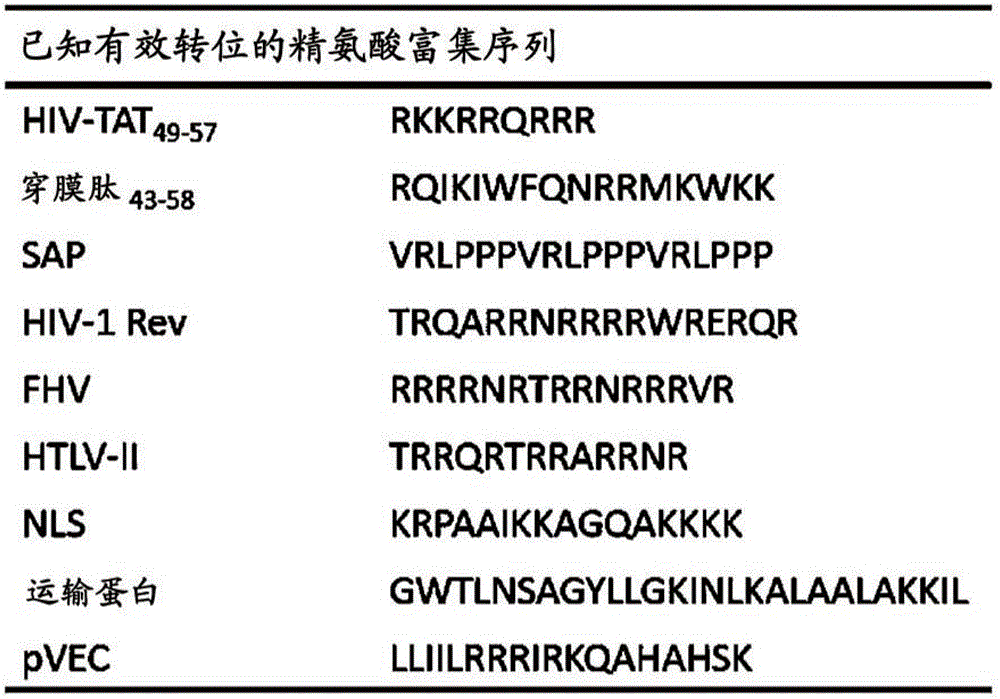
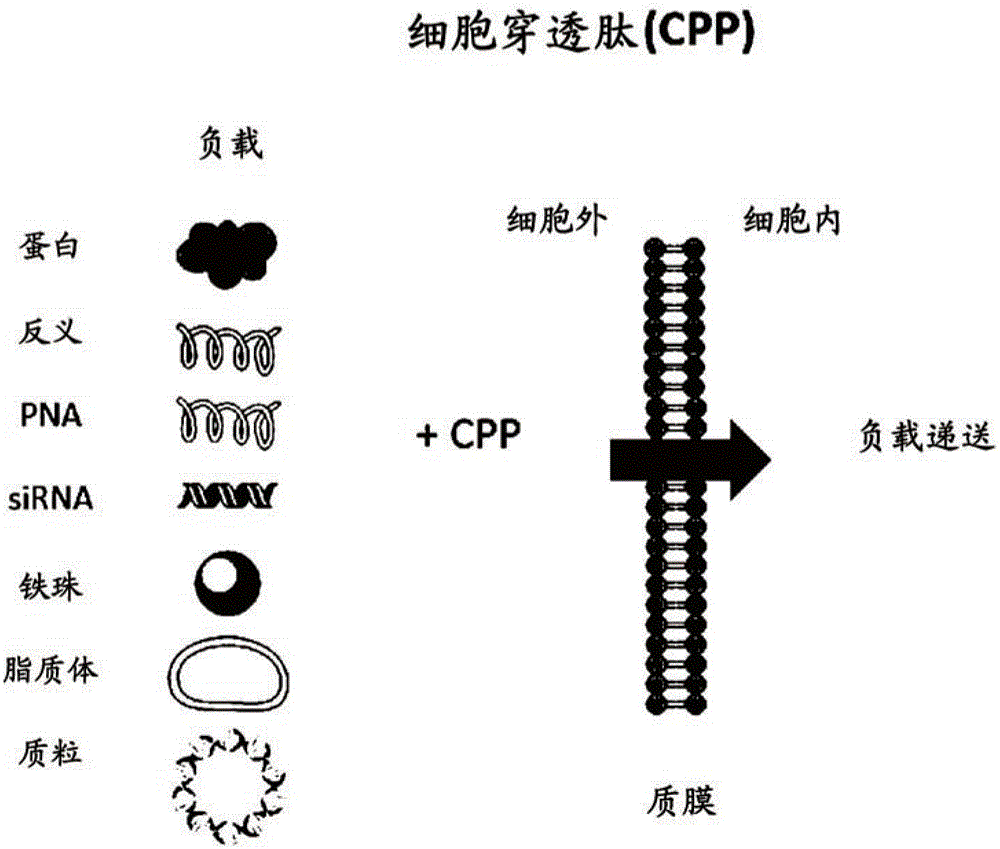
Alpha helix cell-penetrating peptide multimer, preparation method therefor and use thereof
A technology of peptide multimer and multimer, which is applied in the field of α-helical cell penetrating peptide multimer, which can solve the problems of cytotoxicity and rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1: Synthesis of peptide monomers and dimers
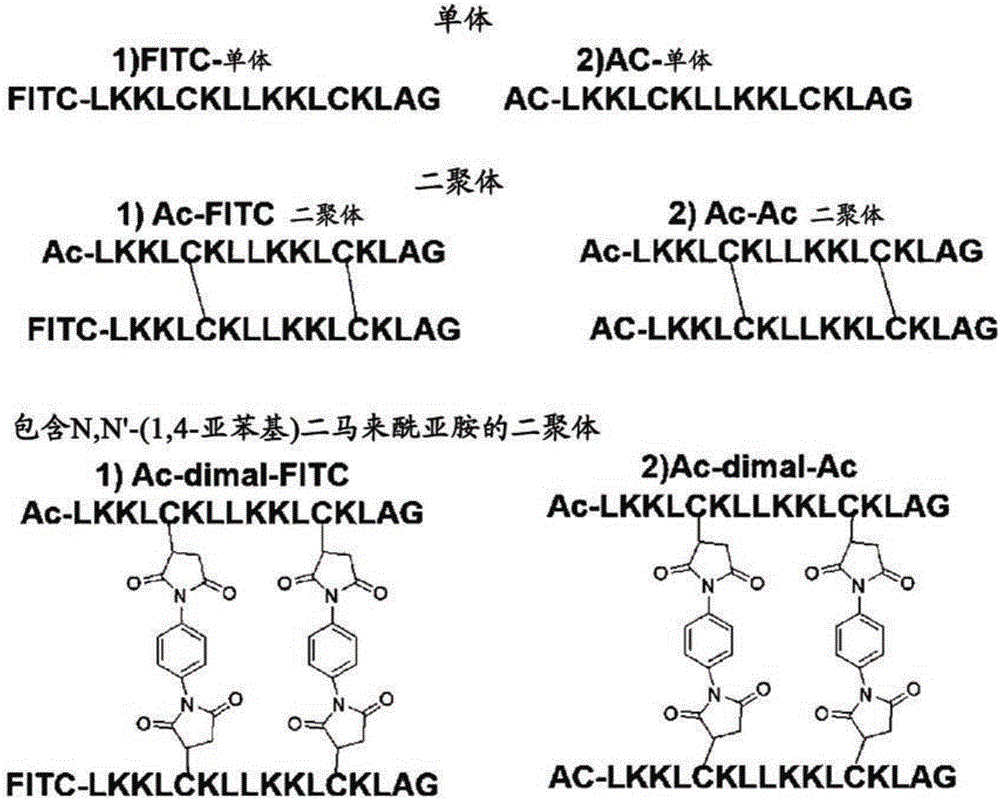
[0131] Monomers LK (LKKLLKLLKKLLKLAG), monomers A (CKKLLKLCKKLLKLAG), B (LKKCLKLLKKCLKLAG), C (LKKLCKLLKKLCKLAG), D (LKKLLKCLKKLLKCAG) and E (LKKLLKLCKKLLKLCG), each with two half-cysteines, were synthesized using solid-phase synthesis method and Fmoc chemistry Amino acid residues, monomer AR (LRRLRLLLRRLLRLAG), wherein all K residues in the amino acid sequence of LK are substituted with R, monomers RA (CRRLLRLCRRRLLRLAG), RB (LRRCLRLLRRRCLRLAG), RC (LRRLCRLLRRLCRLAG), RD (LRRLLRCLRRRLRCAG) and RE(LRRLRLLCRRRLLRLCG), each having two cysteine residues, and the like. Dimeric peptides with disulfides attached to them were obtained by oxidation of purified monomeric peptides under air oxidation conditions (dimers A, B, C, D, E, RA, RB, RC, RD and RE ). Such as image 3 As shown, the Dimal peptide was synthesized using a spacer according to the Michael reaction.
[0132] To observe the characteristics of cell pene...
Embodiment 2
[0134] Example 2: CD (Circular Dichroism) Analysis of Synthesized Peptides
[0135] CD (Circular Dichroism) was used to visualize the secondary structure of the synthesized monomeric, dimer and Dimal peptides. In particular, the dimers show close to 100% α-helical content even in aqueous environments. This α-helical content is very different from the monomer (30% in aqueous solution).
[0136] It is believed that when disulfide bonds exist at positions i and i+7 in one direction of the α-helix, two covalent bonds can maintain high α-helical content by combining the α-helical shape. Like dimeric peptides, Dimal peptides also have a high α-content because they are synthesized using two covalent bonds. However, Dimal peptides have a lower α-helical content than dimeric peptides due to the softness of the molecule.
[0137] Dimeric / Dimal peptides are treated with DTT which is a reducing environment (similar to the cytoplasmic environment). With this treatment it was possible...
Embodiment 3
[0138] Example 3: Cell Penetration Test of Peptides
[0139] The intracellular uptake of three different peptides was compared with each other by FACS experiments at different concentrations of FITC-labeled peptides ( Figure 4 ). At high peptide concentrations (500 nM), dimers (LK-3) and monomers (LK-1 or LK-2) showed high uptake efficiencies (90% or more) with little or no difference. However, at low concentration (10 nM), it was observed that the dimer (LK-3) showed a high uptake efficiency, while the uptake efficiency of the monomer was greatly reduced to less than 10%.
[0140] Specifically, it was observed that the Dimal peptide (LK-4) was taken up even at low concentrations, but its uptake efficiency was about 10-15% lower than that of the dimer (90% or more). The order of cell penetration ability at low concentration and high concentration is: dimer>Dimal>monomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com