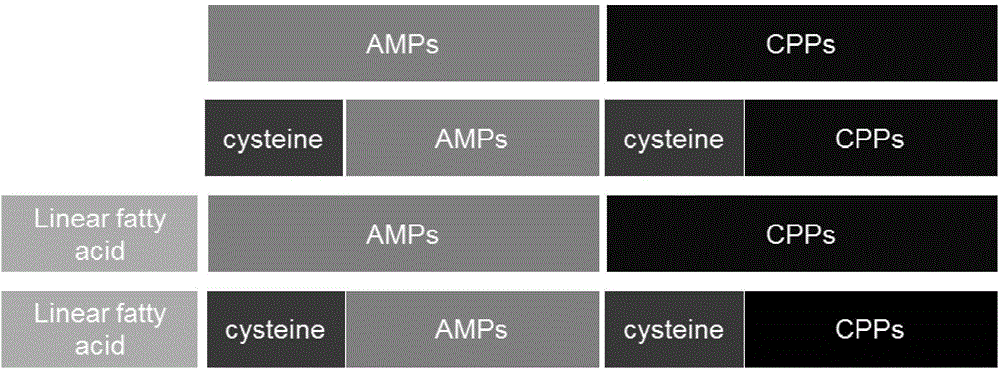
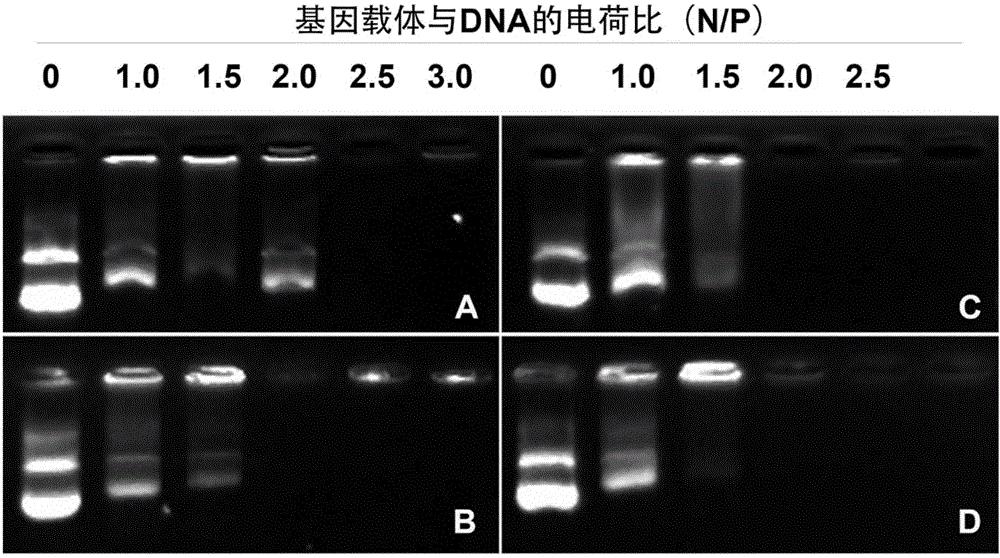
Polypeptide nucleic acid vector, preparation method and uses thereof
A nucleic acid carrier and nucleic acid technology, applied in the field of polypeptide nucleic acid carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] 1. G(LLKK) 3 Synthesis of G-TAT
[0084] According to G(LLKK) 3 Amino acid sequence of G-TAT, Ac-Gly-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Gly-Gly-Arg-Lys-Lys-Arg-Arg-Gln -Arg-Arg-Arg-NH 2 , with Rink-amide resin as the solid phase carrier and HBTU-HOBt as the condensing agent, the target peptide sequence was synthesized using a microwave peptide synthesizer (CEM, USA) using the standard Fmoc strategy. Use 20ml of trifluoroacetic acid: thioanisole: m-cresol: ethanedithiol: water (8.25:0.5:0.5:0.25:0.5, volume ratio) as the lysate, react at 0°C for 30 minutes, and at room temperature for 120 minutes, The peptide is deprotected and cleaved from the resin. The solution was purified by RP-HPLC, RP-HPLC conditions, phase A: 0.05% TFA / water; phase B: 0.05% TFA / 70% ACN / water; column: C8; MALDI-Tof-MS: 2999.84.
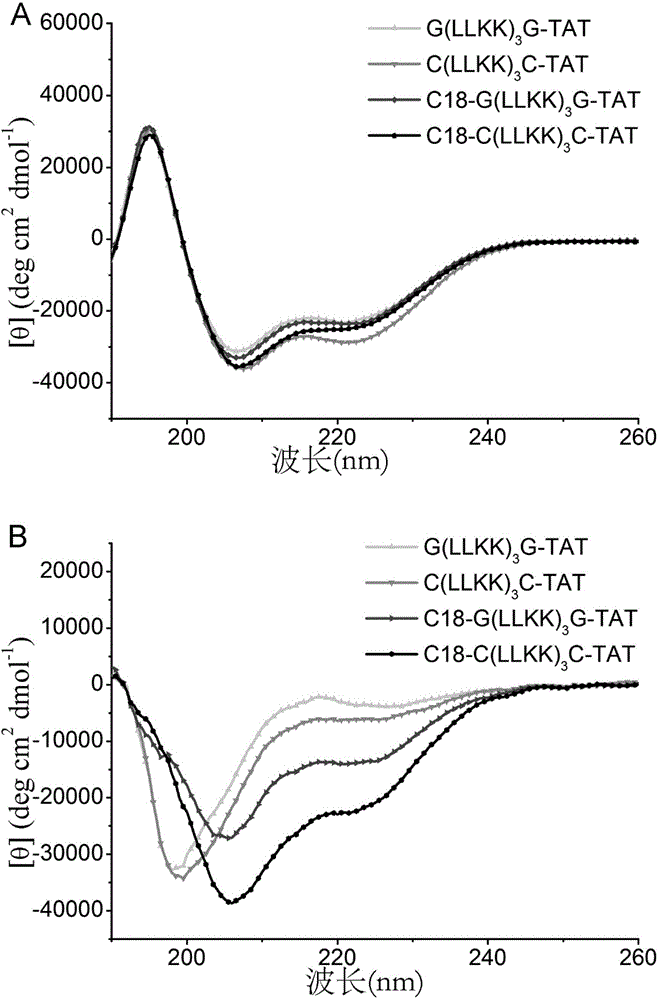
[0085] 2. G(LLKK) 3 Circular Dichroism Characterization of G-TAT
[0086] In order to verify whether the polypeptide can form α-helix, the circular di...
Embodiment 2
[0101] 1. C (LLKK) 3 Synthesis of C-TAT
[0102]According to C (LLKK) 3 Amino acid sequence of C-TAT, Ac-Cys-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Cys-Gly-Arg-Lys-Lys-Arg-Arg-Gln -Arg-Arg-Arg-NH 2 , with Rink-amide resin as the solid phase carrier and HBTU-HOBt as the condensing agent, the target peptide sequence was synthesized using a microwave peptide synthesizer (CEM, USA) using the standard Fmoc strategy. Use 20ml of trifluoroacetic acid: thioanisole: m-cresol: ethanedithiol: water (8.25:0.5:0.5:0.25:0.5, volume ratio) as the lysate, react at 0°C for 30 minutes, and at room temperature for 120 minutes, The peptide is deprotected and cleaved from the resin. The solution was purified by RP-HPLC, RP-HPLC conditions, phase A: 0.05% TFA / water; phase B: 0.05% TFA / 70% ACN / water; column: C8; MALDI-Tof-MS: 3093.26.
[0103] 2. C (LLKK) 3 Circular Dichroism Characterization of C-TAT
[0104] In order to verify whether the polypeptide can form α-helix, the circular ...
Embodiment 3
[0119] 1. C18-G(LLKK) 3 Synthesis of G-TAT
[0120] According to C18-G (LLKK) 3 Amino acid sequence of G-TAT, C18-Gly-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Leu-Leu-Lys-Lys-Gly-Gly-Arg-Lys-Lys-Arg-Arg-Gln -Arg-Arg-Arg-NH 2 (where C18 is stearic acid), using Rink-amide resin as a solid phase carrier, HBTU-HOBt as a condensing agent, and using a standard Fmoc strategy, the target peptide sequence was synthesized using a microwave peptide synthesizer (CEM, USA). Use 20ml of trifluoroacetic acid: thioanisole: m-cresol: ethanedithiol: water (8.25:0.5:0.5:0.25:0.5, volume ratio) as the lysate, react at 0°C for 30 minutes, and at room temperature for 120 minutes, The peptide is deprotected and cleaved from the resin. The solution was purified by RP-HPLC, RP-HPLC conditions, phase A: 0.05% TFA / water; phase B: 0.05% TFA / 70% ACN / water; column: C8; MALDI-Tof-MS: 3225.84.
[0121] 2. C 18 -G(LLKK) 3 Circular Dichroism Characterization of G-TAT
[0122]In order to verify whether the polyp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| helicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com