Irinotecan nano lipid binding preparation and preparation method thereof
A nano-lipid bundle preparation, the technology of irinotecan, is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Enhance tumor-targeted enrichment, long-term circulation, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. Preparation of precursor of irinotecan lipid bundle preparation
[0061] 1. Weigh 1.2g of phospholipid (Germany Lipoid Company) and 0.3g of ethanol, dissolve to obtain solution A.
[0062] 2. Weigh 0.5g irinotecan, 2.5g ethanol, 3.0g Solutol HS15 (German BASF company), 1.8g glycerol, dissolve at room temperature to obtain solution B.
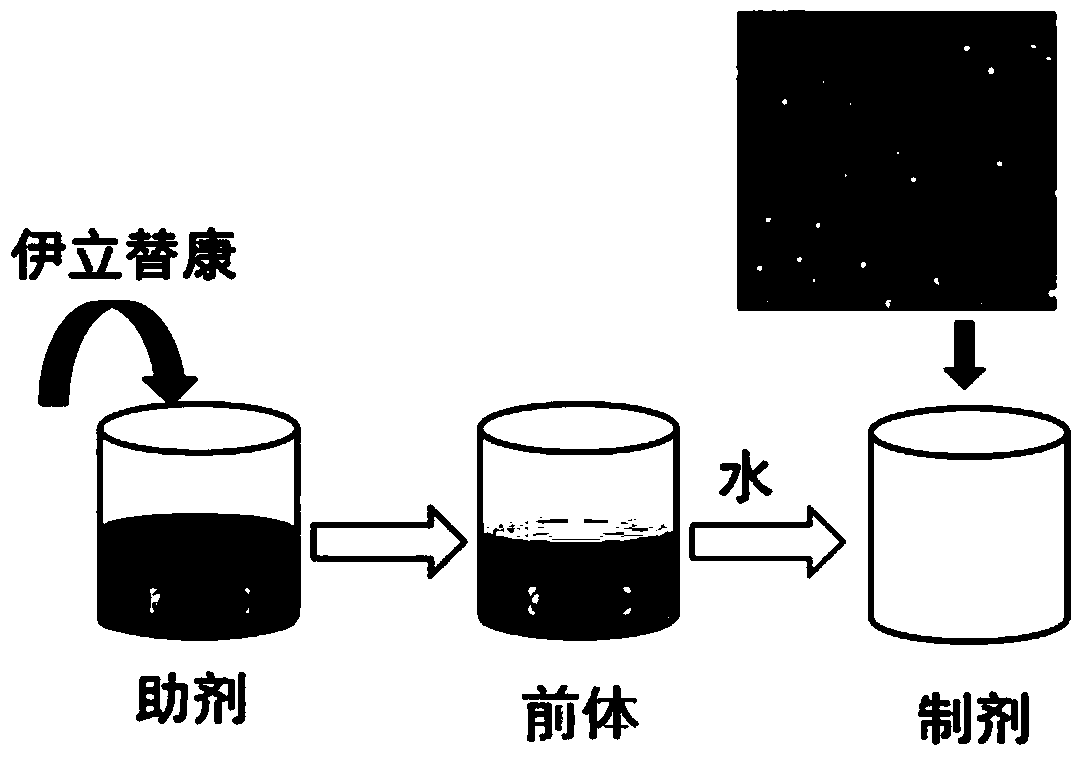
[0063] 3. Mix solution A and solution B, stir and mix at room temperature to obtain a clear and transparent irinotecan lipid bundle preparation precursor, which is protected under nitrogen and sealed for storage. figure 2 Display: The precursor of irinotecan lipid bundle preparation is a clear and transparent liquid.
[0064] Two, the preparation of irinotecan lipid bundle preparation
[0065] Dilute the irinotecan lipid bundle preparation precursor prepared in step 1 with 20 times the volume of physiological saline to obtain irinotecan nano-preparation. image 3 The middle left test tube shows the irinotecan lipid beam formulation...
Embodiment 2
[0070]1. Preparation of precursor of irinotecan lipid bundle preparation
[0071] 1. Weigh 1.2g of phospholipid (Germany Lipoid Company) and 0.3g of ethanol, dissolve to obtain solution A.
[0072] 2. Weigh 0.2g irinotecan, 2.5g ethanol, 3.0g Solutol HS15 (German BASF company), 1.8g glycerol, dissolve at room temperature to obtain solution B.
[0073] 3. Mix solution A and solution B, stir and mix at room temperature to obtain a clear and transparent irinotecan lipid bundle preparation precursor, which is protected under nitrogen and sealed for storage. Similar results figure 2 , The precursor of irinotecan lipid bundle preparation is a clear and transparent liquid.
[0074] Two, the preparation of irinotecan lipid bundle preparation
[0075] Dilute the irinotecan lipid bundle preparation precursor prepared in step 1 with 20 times the volume of normal saline to obtain the irinotecan lipid bundle preparation. Similar results image 3 In the middle left test tube, the irin...
Embodiment 3
[0079] 1. Preparation of precursor of irinotecan lipid bundle preparation
[0080] 1. Weigh 1.2g of phospholipid (Germany Lipoid Company) and 0.3g of ethanol, dissolve to obtain solution A.
[0081] 2. Weigh 0.8g irinotecan, 2.5g ethanol, 3.0g Solutol HS15 (German BASF company), 1.8g glycerol, dissolve at room temperature to obtain solution B.
[0082] 3. Mix solution A and solution B, stir and mix at room temperature to obtain a clear and transparent irinotecan lipid bundle preparation precursor, which is protected under nitrogen and sealed for storage. Similar results figure 2 , The precursor of irinotecan lipid bundle preparation is a clear and transparent liquid.
[0083] Two, the preparation of irinotecan lipid bundle preparation
[0084] Dilute the irinotecan lipid bundle preparation precursor prepared in step 1 with 20 times the volume of normal saline to obtain the irinotecan lipid bundle preparation. Similar results image 3 In the middle left test tube, the iri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com