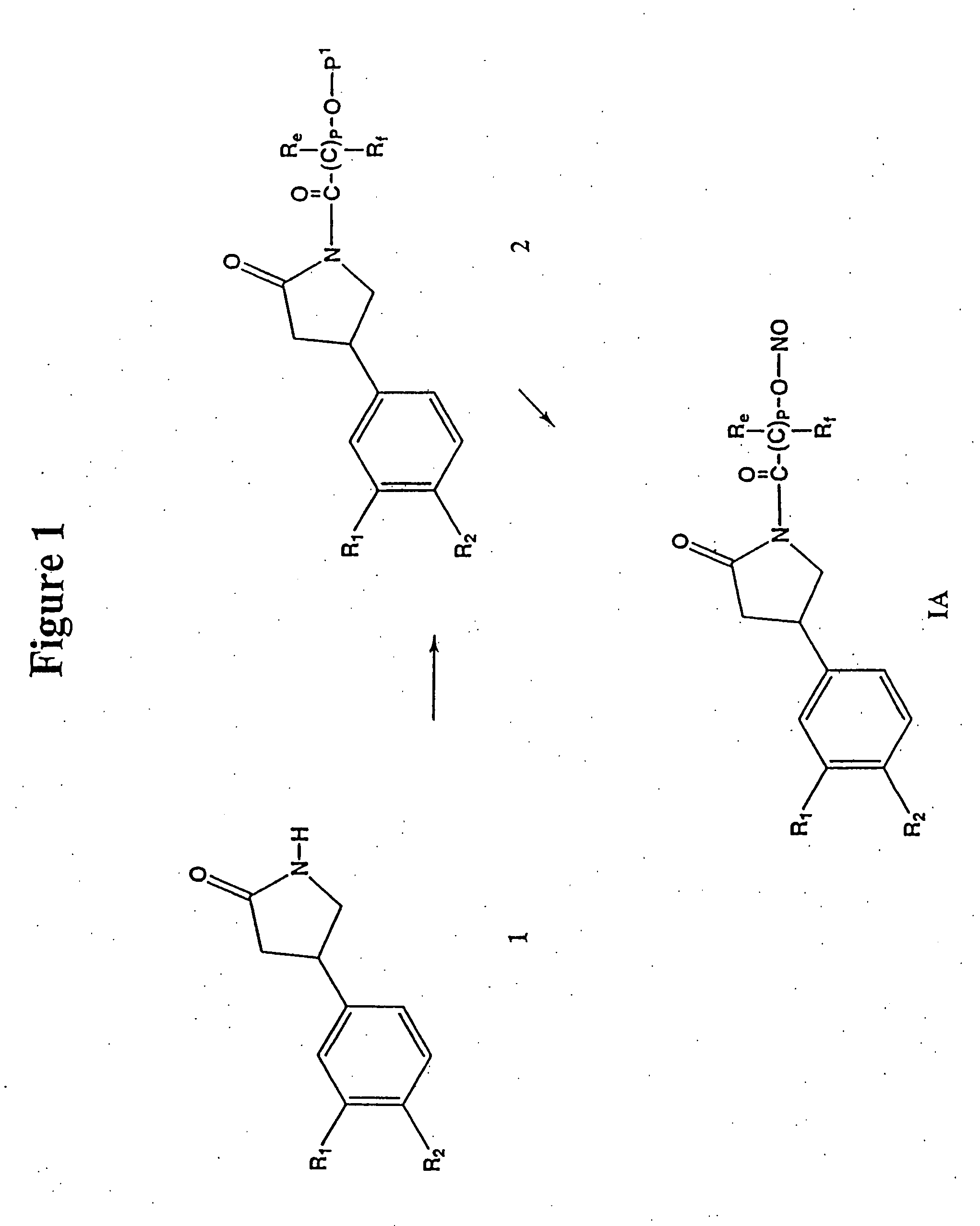
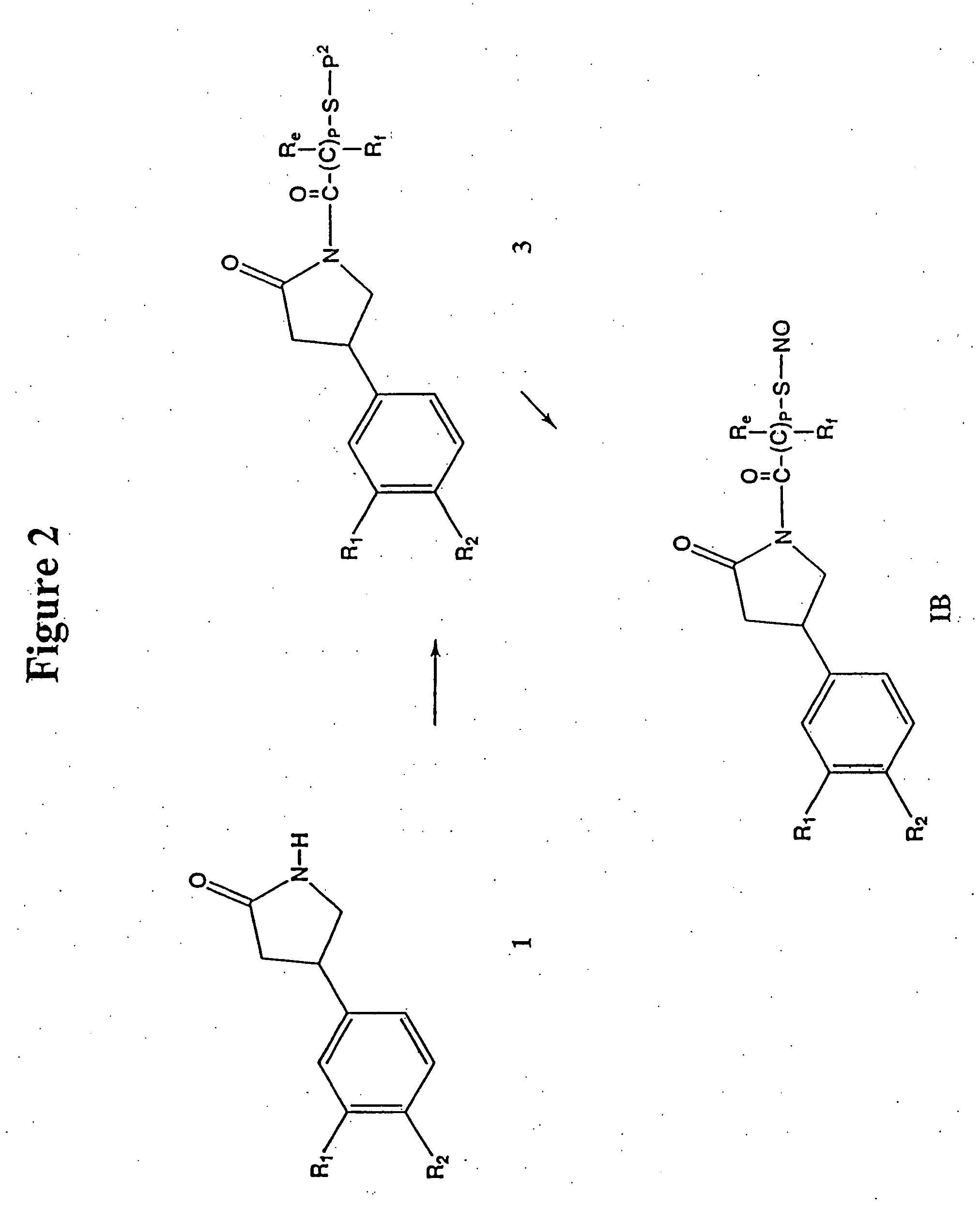
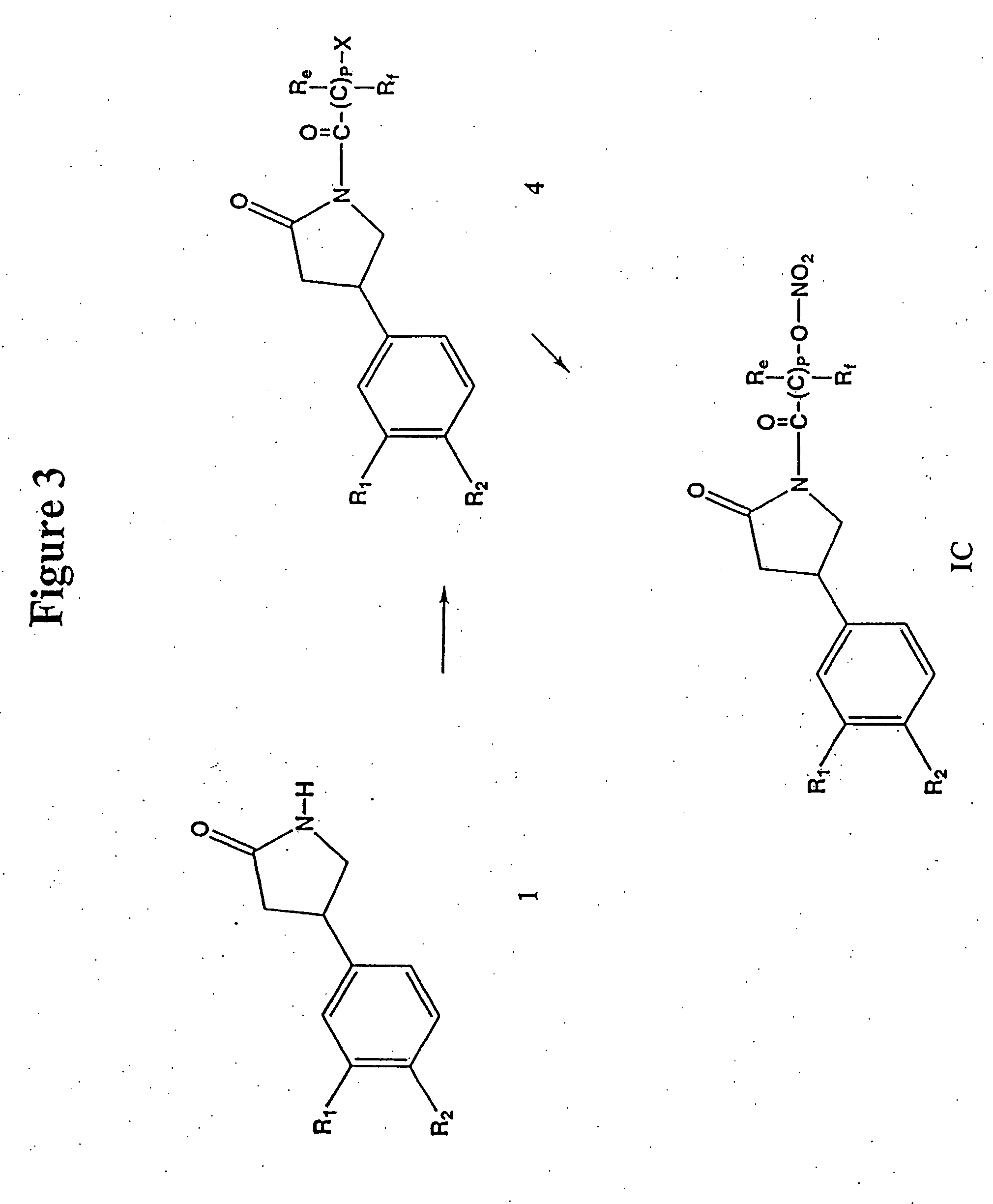
Phosphodiesterase inhibitors and nitric oxide donors, compositions and methods of use
a technology of phosphodiesterase inhibitors and nitric oxide donors, applied in the direction of drug compositions, ester active ingredients, cardiovascular disorders, etc., can solve the problems that the effects of modified phosphodiesterase inhibitors, which are directly or indirectly linked with nitric oxide adducts, have not been previously investigated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2,6-bis(diethyl(3-methyl-3-(nitrosothio)butyric acid ester)amino)-4,8-dipiperidinopyrimido-[5,4-d]-pyrimidine
1a. 3-Methyl-3(2,4,6-trimethoxyphenylmethylthio)butyric acid
[0386] To a solution of 3-mercapto-3-methylbutyric acid (Sweetman et al, J. Med. Chem., 14:868 (1971)) (4.6 g, 34 mmol) in methylene chloride (250 ml) under nitrogen and cooled over ice / salt to 5° C. (internal temperature) was added trifluoroacetic acid (82 g, 0.72 mol). No significant temperature rise was noted during the addition. To this was then added dropwise a solution of 2,4,6-trimethoxybenzyl alcohol (Munson et al, J. Org. Chem., 57:3013 (1992)) (6.45 g. 32 mmol) in methylene chloride (150 ml) such that the reaction temperature does not rise above 5° C. After the addition was complete, the mixture was stirred for an additional 5 minutes at 5° C. and the volatiles were removed in vacuo (toluene or ethyl acetate can be used to assist in the removal of volatile material). The residue was partitioned between di...
example 2
1-(4-{{1,3-benzodioxol-5-methyl)amino)-6-chloro-2-quinazolinyl)-4-piperidine-carboxylic ethyl-(3-methyl-3(nitrosothiol)butyramide) thioester hydrochloride
2a. 3-Methyl-3(thioacetyl)butyric acid
[0390] To a solution of 3-mercapto-3-methylbutyric acid (Sweetman et al, J. Med. Chem., 14:868 (1971)) (1.03 g, 7.7 mmol) in pyridine (1.6 ml) was added acetic anhydride (1.57 g, 15.4 mmol) and the reaction mixture was stirred at room temperature over night. The reaction mixture was slowly added to a 0° C. solution of 1 N HCl (20 ml) then water (10 ml) was added and the reaction mixture was stirred at room temperature for 20 hours. The solution was extracted with diethyl ether and the organic phase was washed with brine and then dried over anhydrous sodium sulfate. The solvent was evaporated in vacuo, and the residue was purified by flash chromatography on silica gel eluting with ethyl acetate / hexane (1:4) to give the title compound (0.791 g, 58% yield). (CDCl3, 300 MHz) δ 1.55 (s, 6H), 2.25 ...
example 3
In Vitro Comparative Relaxation Responses
[0398] Human corpus cavernosum tissue biopsies were obtained at the time of penile prosthesis implantation from impotent men. The tissue was maintained in a chilled Krebs-bicarbonate solution prior to assay. The tissue was cut into strips of 0.3×0.3×1 cm and suspended in organ chambers for isometric tension measurement. Tissues were incrementally stretched until optimal isometric tension for contraction was obtained. Once this was achieved, the tissues were contracted with phenylephrine (7×10−7 M) and once a stable contraction was achieved, the tissues were exposed to either dipyridamole or the compound of Example 1 (10−6 to 3×10−5 M) by cumulative additions to the chamber. At the end of the experiment, papaverine (10−4 M) was added to obtain maximal relaxation. FIG. 58 shows that the compound of Example 1 at doses of 10 μM and 30 μM was more efficacious in relaxing the phenylephrine-induced contraction than was an equimolar dose of the phos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com