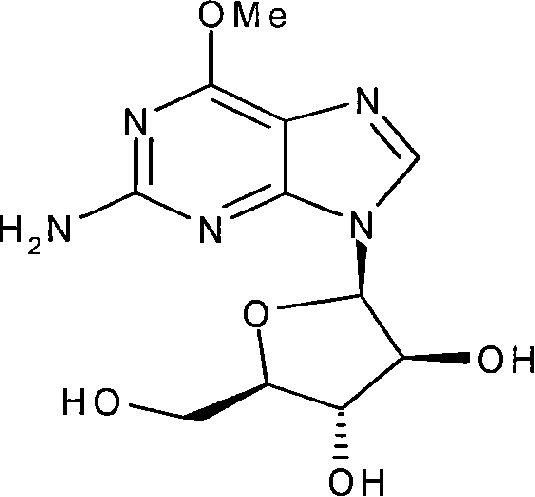
Method for synthesizing nelarabine
A synthesis method and a technology for chemical synthesis, applied in the field of medicine, can solve the problems of strict equipment requirements, high price, low nerabine yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
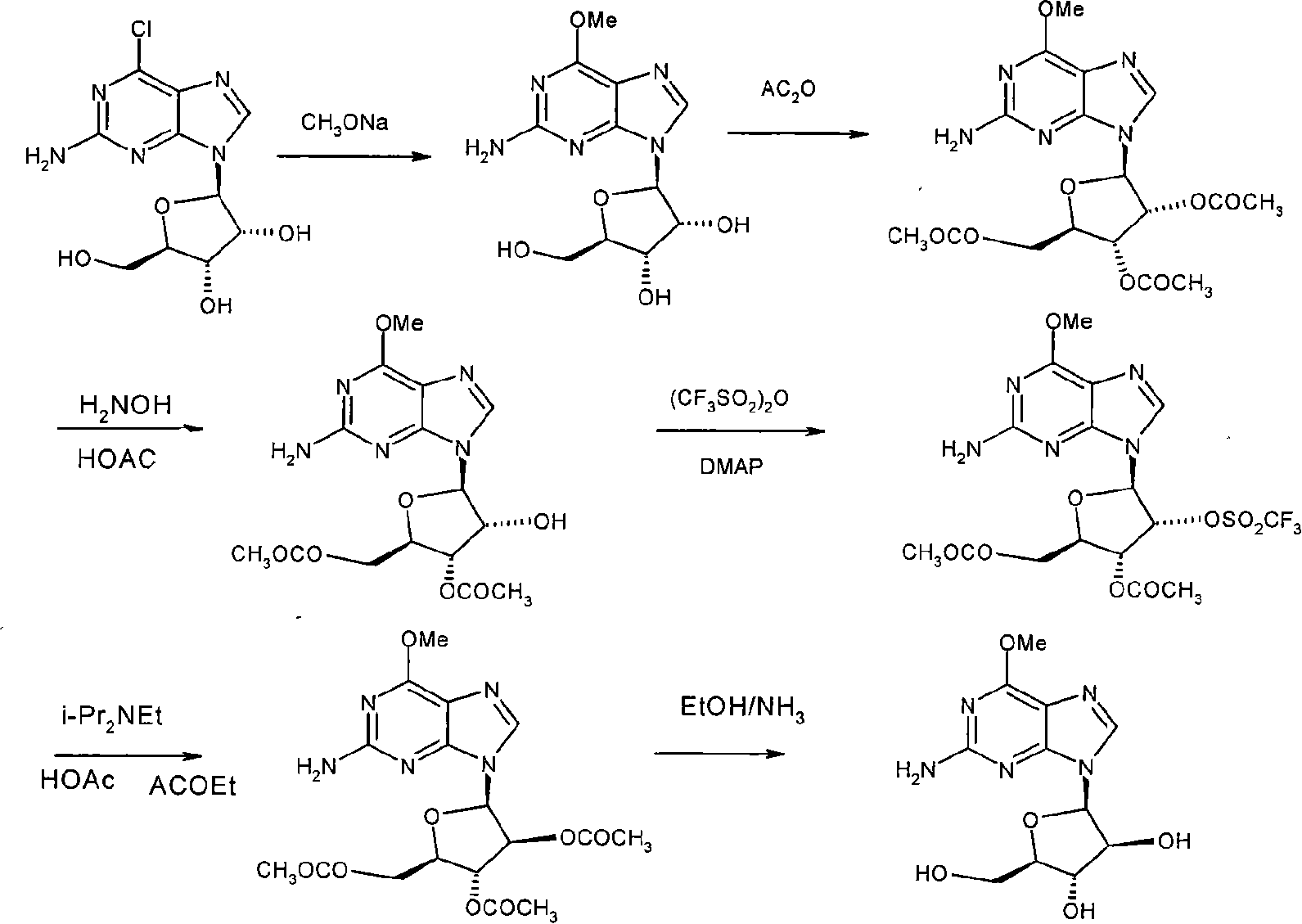
[0073] The preparation method of 6-methoxyguanosine is as follows: take 248.0 grams of sodium methoxide and add it to methanol, stir to obtain a white turbid liquid, add 310 grams of 6-chloroguanosine material to dissolve rapidly to obtain a light yellow solution, and then add Precipitate a white solid, heat to reflux, continue the reaction, cool to room temperature, carefully add hydrochloric acid solution dropwise with stirring to neutralize to pH=8-9, remove the solvent and add distilled water to the remaining pale yellow viscous material, dissolve under stirring at 70°C, Then add activated carbon, hot filter, filtrate, naturally cool and crystallize, filter to obtain colorless crystalline solid, vacuum dry to obtain 196.7 g of white solid, namely 6-methoxyguanosine. mp: 133-135°C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, starting Rf=0.45.
[0074] The preparation method of 2', 3', 5'-tri-O-acetyl-6-methoxyguanosine is as follows: dissolve 195.0 grams of 6-methoxyg...
Embodiment 2
[0082] Wherein the preparation method of 6-methoxyguanosine is: get 128 grams of sodium methylate and join in the methanol, stir, obtain white turbid liquid, add 160 grams of 6-chloroguanosine materials and dissolve rapidly, obtain light yellow solution, then again Precipitate a white solid, heat to reflux, continue the reaction, cool to room temperature, carefully dropwise add hydrochloric acid solution to neutralize to PH = 8-9 while stirring, add distilled water to the remaining light yellow viscous substance after removing the solvent, dissolve under stirring at 70°C, Activated carbon was then added, hot filtered, and the filtrate was naturally cooled and crystallized, filtered to obtain a colorless crystalline solid, and vacuum-dried to obtain 102.6 g of a white solid, namely 6-methoxyguanosine. mp: 133-135°C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, starting material Rf=0.45.
[0083] Wherein 2′, 3′, 5′-tri-O-acetyl-6-methoxyguanosine is prepared by dissolving 1...
Embodiment 3
[0091] Wherein the preparation method of 6-methoxyguanosine is: get 800 grams of sodium methylate and join in methanol, stir, obtain white turbid liquid, add 1000 grams of 6-chloroguanosine materials and dissolve rapidly, obtain light yellow solution, then again Precipitate a white solid, heat to reflux, continue the reaction, cool to room temperature, carefully dropwise add hydrochloric acid solution to neutralize to PH = 8-9 while stirring, add distilled water to the remaining light yellow viscous substance after removing the solvent, dissolve under stirring at 70°C, Activated carbon was then added, hot filtered, and the filtrate was naturally cooled and crystallized, filtered to obtain a colorless crystalline solid, and vacuum-dried to obtain 598.8 g of a white solid, namely 6-methoxyguanosine. mp: 133-135°C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, starting material Rf=0.45.
[0092]Wherein, the preparation method of 2', 3', 5'-tri-O-acetyl-6-methoxyguanosine is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com