Dinucleotide crystals
A technology for crystallization and uridine, applied in the field of P1-P4-tetraphosphate or a pharmaceutically acceptable salt thereof, to prepare the crystallization, can solve the problems that it cannot be used as a practical method and the synthesis yield is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
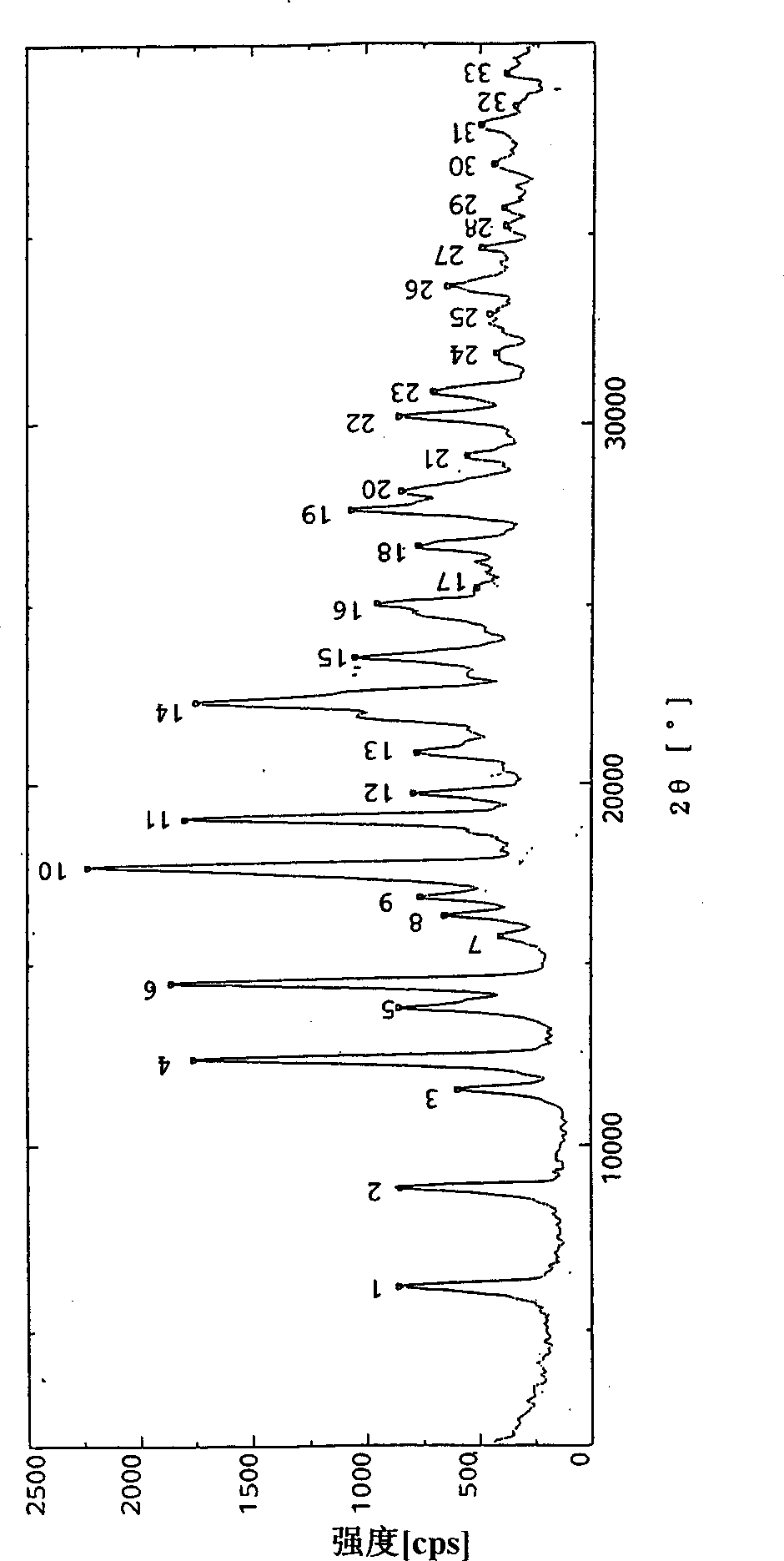
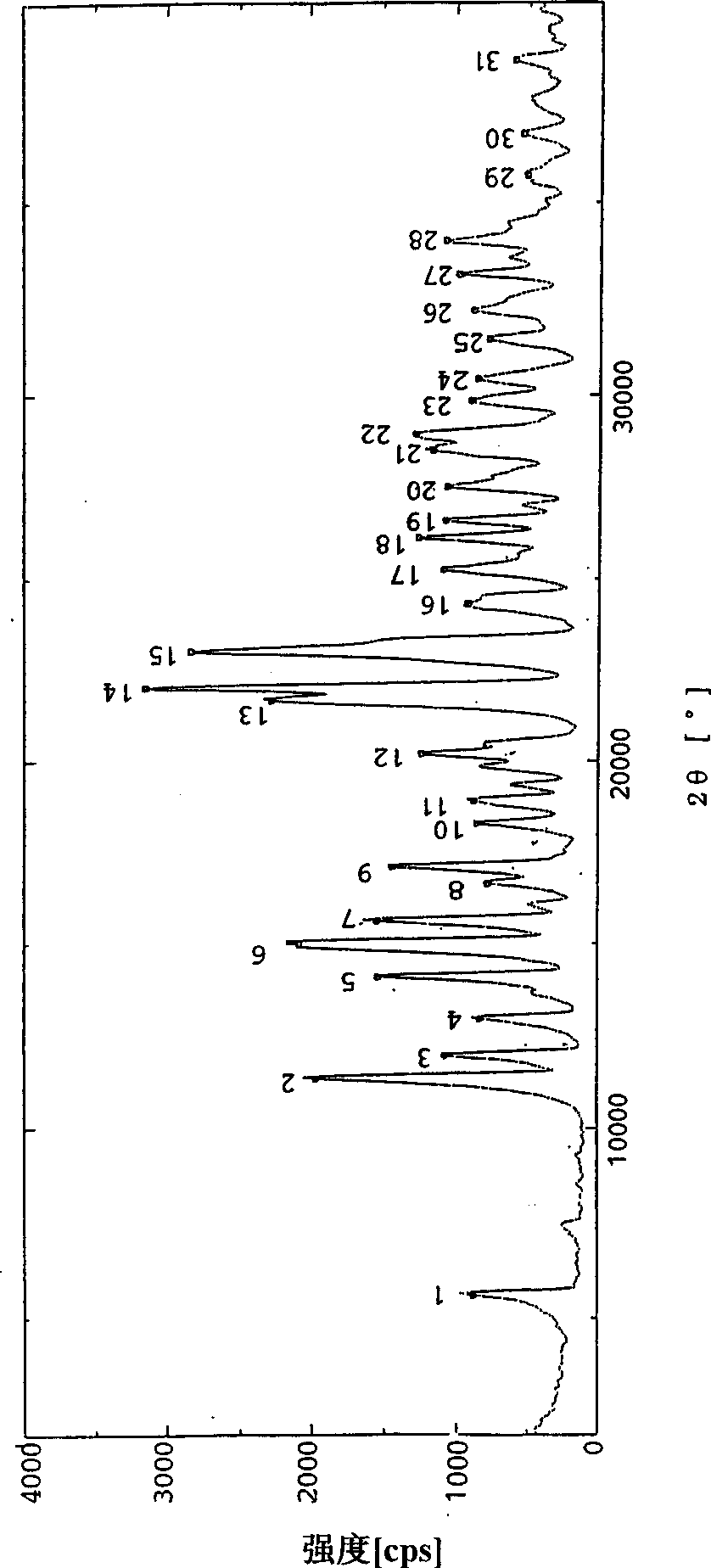
Image
Examples
Embodiment 1
[0044] The present invention will be described in more detail through examples below, but the examples do not limit the present invention. Example 1 Preparation of dCP4U·4Na crystals (1) Method using DCC
[0045] dCP4U was prepared by the application of UTP, dCMP and DCC by conventional methods as described in WO 98 / 34942. Reactions were performed on a 20 mmol scale.
[0046] The dCP4U solution thus obtained was diluted with water to adjust to a total volume of 1000 ml, and the diluted solution was loaded on a column of a moderately basic anion exchange resin (AMBERLITE IRA-67, product of Rohm & Hass Co.). Fractions containing dCP4U were collected by sequentially eluting with water, 0.18M aqueous hydrochloric acid solution, and 0.005M aqueous hydrochloric acid solution containing 0.5M sodium chloride.
[0047] The thus obtained dCP4U fraction (4000 ml) was applied to a column with chromatography-grade activated carbon (Taiko Granular Activated Charcoal SGP, product of Futamu...
Embodiment 2
[0096] (13) 39.3 Example 2: Synthesis of dCP4U initiated by UMP
[0097] Formamide (2.5 ml) and pyridine (7.6 ml) were added to triethylamine salt (10 mmol) of anhydrous pyrophosphoric acid (TEA-PPi), followed by stirring the resulting mixture. To tributylamine salt of anhydrouridine 5'-monophosphate (UMP-TBA) (10 mmol) in another vessel was added DMAC (3.6 ml), dioxane (3.2 ml), and tributyl Amine (3.3ml) was stirred and DPC (2.3ml) was added dropwise thereto. The mixture was stirred at room temperature for 1 hour, whereby UMP-DPP was formed, which was added to the previously prepared dehydrated TEA-PPi solution described above. The reaction mixture was stirred at room temperature for 1 hour, resulting in UTP. To the tributylamine salt of 2'-deoxycytidine 5'-monophosphate (TBA-dCMP) (4.9 g, 10 mmol) in another vessel was added DMAC (7.2 ml), thereby forming a suspension, And DPC (2.2ml, 1.1 equiv) was added to the suspension. The resulting mixture was stirred for 40 minut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com