Method for preparing 2', 3'2-dideoxycytidine
A technology of dideoxycytidine and its production method, which is applied in the field of anti-AIDS drugs and can solve problems such as low product yield and complex process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] A production method of 2', 3'-dideoxycytidine, comprising the following steps in sequence:
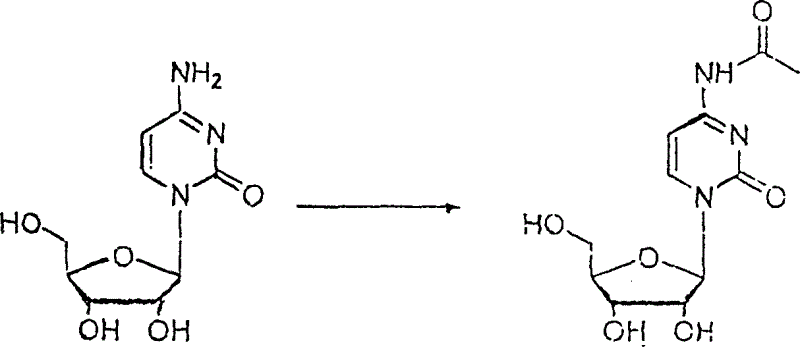
[0021] ①Reaction of cytidine and acetic anhydride to generate 4-N-acetyl cytidine: 10 grams of cytidine and 12-30ml (can be 12, 18, 25, 30ml) of acetic anhydride are heated under reflux for 10-20 Hours (Example 10, 15, 20 hours), then remove the solvent under reduced pressure, then add toluene 50×2 to azeotropically remove the residue to obtain a colorless powder product. Reaction formula:
[0022]
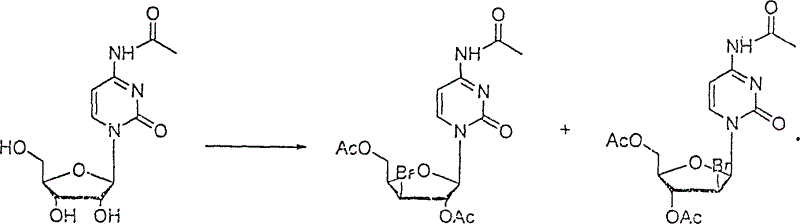
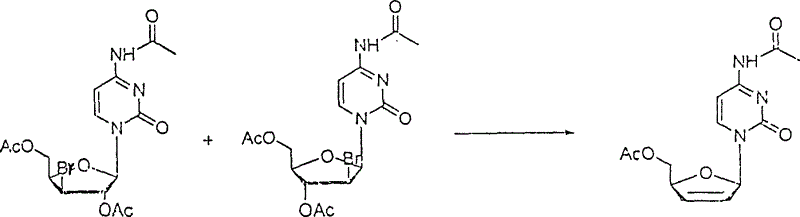
[0023] ②The 4-N-acetyl cytidine obtained above and the saturated acetic acid solution of hydrogen bromide are reacted under the condition of acetic anhydride as a catalyst to generate a brominated mixture, which is a pair of isomers: 10 grams of 4-N-acetyl Cytidine, add 5-15ml (Example 5, 10, 15ml) of saturated acetic acid solution of hydrogen bromide and 5-15ml (Example 5, 10, 15ml) of freshly distilled acetic anhydride, heat the oil bath to 40-60°C (Example 40, 50, 60°C), rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com