Novel method for synthesizing 5-carboxyl-2'-deoxycytidine
A deoxycytidine and carboxyl technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of expensive raw materials, etc., and achieve simple synthesis methods, high product yields, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
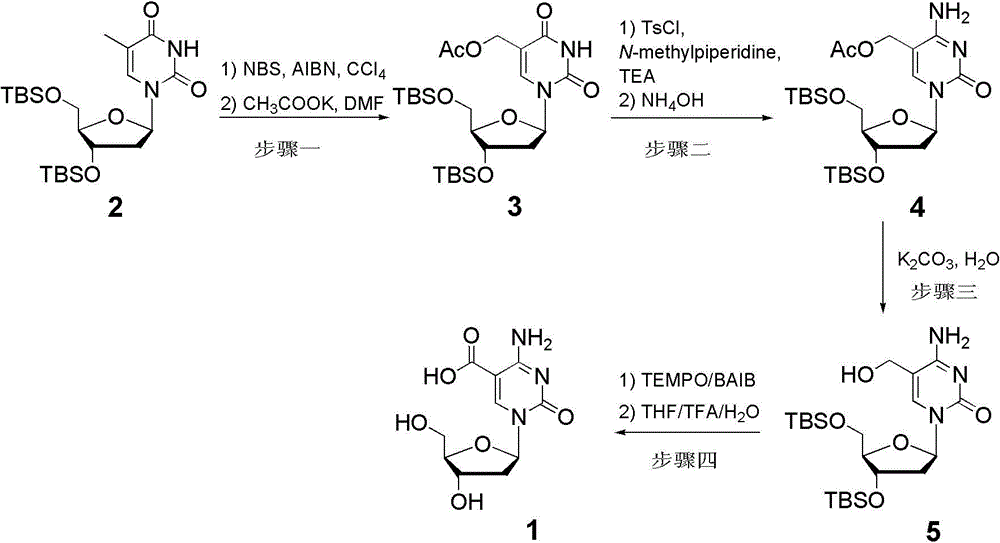
[0011] 1) 3',5'-di-tert-butyldimethylsilyl-5-acetylmethyl-2'-deoxyuridine ( 3 ) synthesis: under argon protection, the 2 (12 g, 25.4 mmol) was dissolved in dry carbon tetrachloride (200 mL), and recrystallized NBS (5.42 g, 30.4 mmol) and AIBN (100 mg, 0.61 mmol) were added at 60 °C, and the temperature was raised to 80 °C for 0.5 h, then added a second batch of recrystallized NBS (5.42 g, 30.4 mmol) and AIBN (100 mg, 0.61 mmol) into the reaction flask, and continued to stir for 1 h. Cool to room temperature, add chloroform (100 mL) to dilute the reaction solution, then wash with saturated brine (200 mL × 2), collect the organic phase, wash with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain crude 5-bromomethyl-2'-deoxyuridine. Subsequently, under the protection of argon, dry N , N - Dimethylformamide (20 mL) and potassium acetate (6.2 g, 63.5 mmol), heated to 40 °C for 0.5 hours. Cool to room temperature, add ethyl acetate (200 mL) to dilute the...
Embodiment 2
[0016] 1) 3',5'-di-tert-butyldimethylsilyl-5-acetylmethyl-2'-deoxycytidine ( 4 ) synthesis: under argon protection, the 3 (4.22 g, 8 mmol) dissolved in dry tetrahydrofuran (80 mL), added at 0 °C N - Methylpiperidine (960 mg, 9.6 mmol) triethylamine (2.44 mL, 17.6 mmol) and p-toluenesulfonyl chloride (3.8 g, 20 mmol). After reacting for 4 hours, 28% concentrated ammonia water (20 mL) was added at 0 °C, and the temperature was raised to 20 °C for 0.5 hour. Add ethyl acetate (400 mL) to dilute the reaction solution, then wash with saturated brine (400 mL×2), collect the organic phase, wash with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. Column chromatography separation (dichloromethane: methanol = 40: 1) to obtain a white solid ( 4 ) 3.03 g, yield 72%;
[0017] 2) 5-Carboxy-2′-deoxycytidine 1 The synthesis of 5 (2.91 g, 6 mmol) was dissolved in acetonitrile (30 mL), water (15 mL) was added, followed by TEMPO (94 mg, 0.6 mmol) and BAIB (3.86 g, 12 mmol...
Embodiment 3
[0019] 1) 3',5'-di-tert-butyldimethylsilyl-5-acetylmethyl-2'-deoxycytidine ( 4 ) synthesis: under argon protection, the 3 (4.22 g, 8 mmol) dissolved in dry dichloromethane (80 mL), added at 0 °C N - Methylpiperidine (960 mg, 9.6 mmol) triethylamine (2.44 mL, 17.6 mmol) and p-toluenesulfonyl chloride (4.56 g, 24 mmol). After reacting for 2 hours, 20% concentrated ammonia water (20 mL) was added at 0 °C, and the temperature was raised to 20 °C for 1 hour. Add ethyl acetate (400 mL) to dilute the reaction solution, then wash with saturated brine (400 mL × 2), collect the organic phase, wash with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. Column chromatography separation (dichloromethane: methanol = 40: 1) to obtain a white solid ( 5 ) 2.94 g, yield 70%;
[0020] 2) 5-carboxy-2′-deoxycytidine ( 1 ) synthesis: the 5 (2.91 g, 6 mmol) was dissolved in acetonitrile (30 mL), then TEMPO (282 mg, 1.8 mmol) and BAIB (5.76 g, 18 mmol) were added and reacted at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com