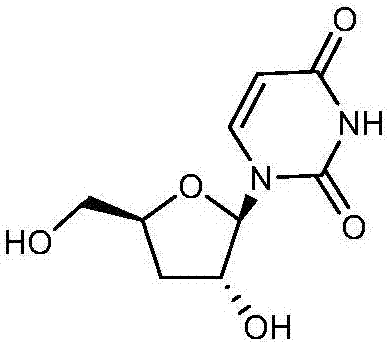
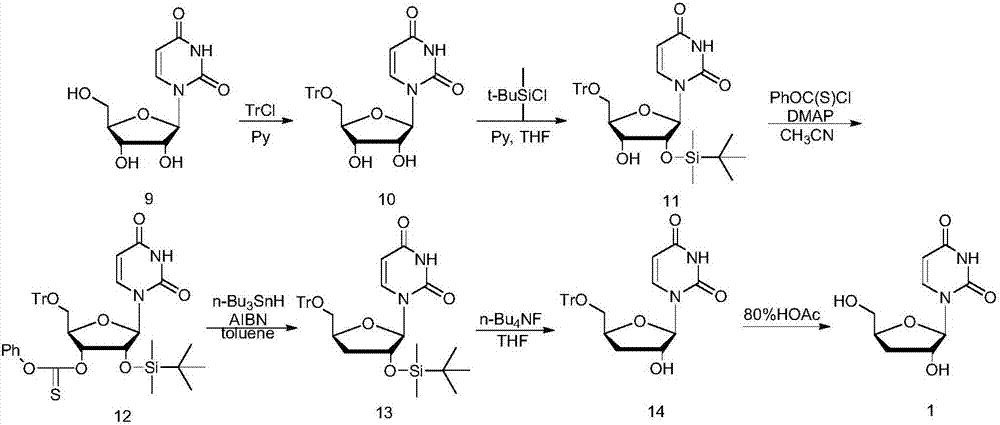
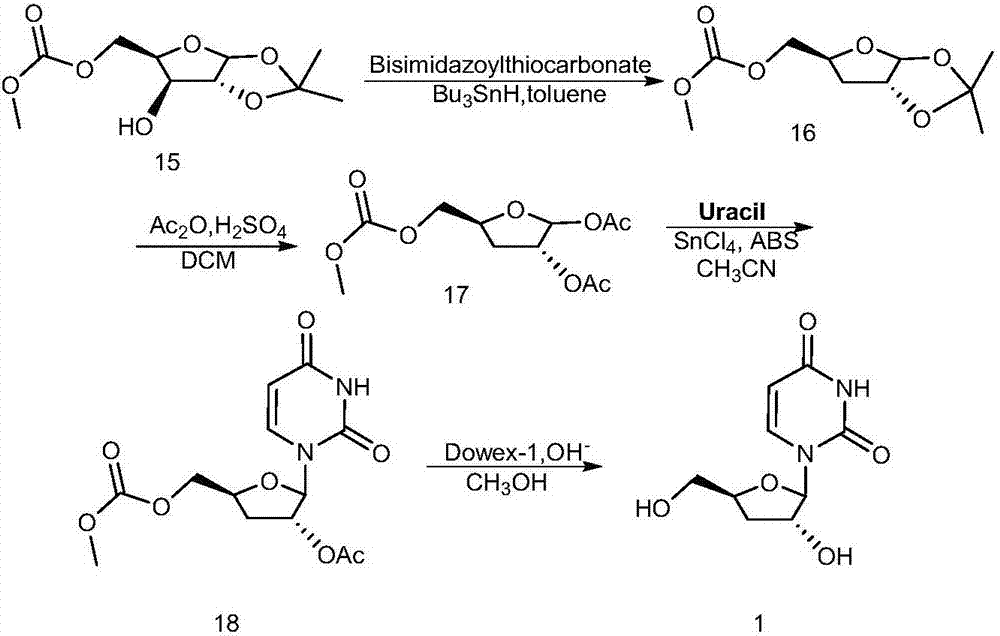
Preparation method of 3'-deoxyuridine
A technology of deoxyuridine and lithium hydroxide, applied in the field of preparation of 3'-deoxyuridine, can solve the problems of waste of precious metals, low industrial production feasibility, high risk, etc., and achieves avoiding strict conditions and expensive costs, It is very easy to industrialize large-scale production and avoid the effect of thiocarbonyl reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation example of compound 4
[0034]
[0035] Add 50.0g of compound 3 powder, 250mL of acetonitrile and 25mL of acetic anhydride into a 500mL three-neck flask, stir and reflux for 3h, cool to room temperature and filter with suction, wash the solid twice with acetonitrile, and dry the filter cake to obtain 56.3g of compound 4, the yield is 96.0 %.
[0036] 1 H NMR (400MHz, DMSO-d 6 ):δ=10.88(s,1H,N-H),8.42(d,1H,J=7.5Hz,6-H,D 2 O exchange), 7.20 (d, 1H, J = 7.5Hz, 5-H), 5.78 (d, 1H, J = 2.8Hz, 1'-H), 5.49 (d, 1H, J = 4.6Hz, 2' -OH,D 2 O exchange), 5.18 (t, 1H, J = 5.0Hz, 5'-OH, D 2 O exchange), 5.06 (d, 1H, = 5.4Hz, 3'-OH, D 2 O exchange), 4.00-3.88 (m, 3H, 2', 3', 4'-H), 3.77-3.56 (m, 2H, 5', 5"-H), 2.10 (s, 3H, CH 3 C=O). 13 CNMR (101MHz, DMSO-d 6 )δ171.00 (COCH 3 ),162.27(4-C),154.65(2-C),145.35(6-C),95.14(1'-C),90.10(5-C),84.15(4'-C),74.48(2' -C),68.62(3'-C),59.87(5'-C),24.33(CH 3 ).
Embodiment 2
[0037] Embodiment 2: the preparation example of compound 5
[0038]
[0039] Add 50.0 g of compound 4 and 750 mL of acetonitrile into a 2 L three-necked flask, raise the temperature to 65° C., and add a solution of 40 mL of AcBr dissolved in 250 mL of acetonitrile. React at 60-65°C for 1h. Cool to room temperature and spin dry. Dissolved in DCM and washed twice with water, Na 2 SO 4 Dry, spin dry, reflux with 500ml DCM to suspend for 60min, freeze to crystallize after cooling to room temperature, filter with suction, and dry to obtain 57.8g of white crystals of compound 5 with a yield of 76.4%.
[0040] 1 H NMR (400MHz, CDCl 3 )δ=10.25(brs,1H,NHAc),8.14(d,1H,6-H,J=7.6Hz),7.53(d,1H,5-H,J=7.6Hz),5.97(d,1H, 1'-H,J=1..1Hz),5.49(d,1H,2'-H),4.60-4.34(m,4H,3',4',5',5"-H),2.29, 2.17,2.14(3s,9H,3*CH 3 CO). 13 C NMR (101MHz, CDCl 3 )δ171.19, 170.48, 168.98(3*C=O), 163.33(4-C), 154.82(2-C), 144.14(6-C), 96.69(1'-C), 90.80(5-C), 82.54(4'-C),79.67(2'-C),64.81(5'-C),48.83(3'-C...
Embodiment 3
[0041] Embodiment 3: the preparation example of compound 6
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com