Serum-free culture medium for mesenchymal stem cells and application thereof
A serum-free medium and mesenchymal stem cell technology, applied in the field of mesenchymal stem cell serum-free medium, can solve the limitations of mesenchymal stem cell research and application, cell productivity, limited number of passages, and limit mesenchymal stem cell application, etc. problems, to achieve the effect of eliminating instability, maintaining differentiation potential, and high-efficiency biological characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The serum-free medium for mesenchymal stem cells provided in this example consists of the following components: basal medium and additional components, the basal medium is DMEM / F12, and the additional components are recorded as MAX1 components, MAX1 components and DMEM / F12 The specific material composition is as follows:
[0033] (1) The components contained in the MAX1 component and the content of each component are (based on the volume of the MAX1 component):
[0034]
[0035] (2) The components contained in the DMEM / F12 component and the content of each component are (based on the volume of the DMEM / F12 component):
[0036]
[0037]
[0038] In this example, the basal medium DMEM / F12 can also be purchased directly from the reagent company.
[0039] In this example, the serum-free medium for mesenchymal stem cells was prepared with DMEM / F12 and MAX1 at a volume ratio of 1000mL: 80mL, and the preparation method was prepared by conventional methods.
Embodiment 2
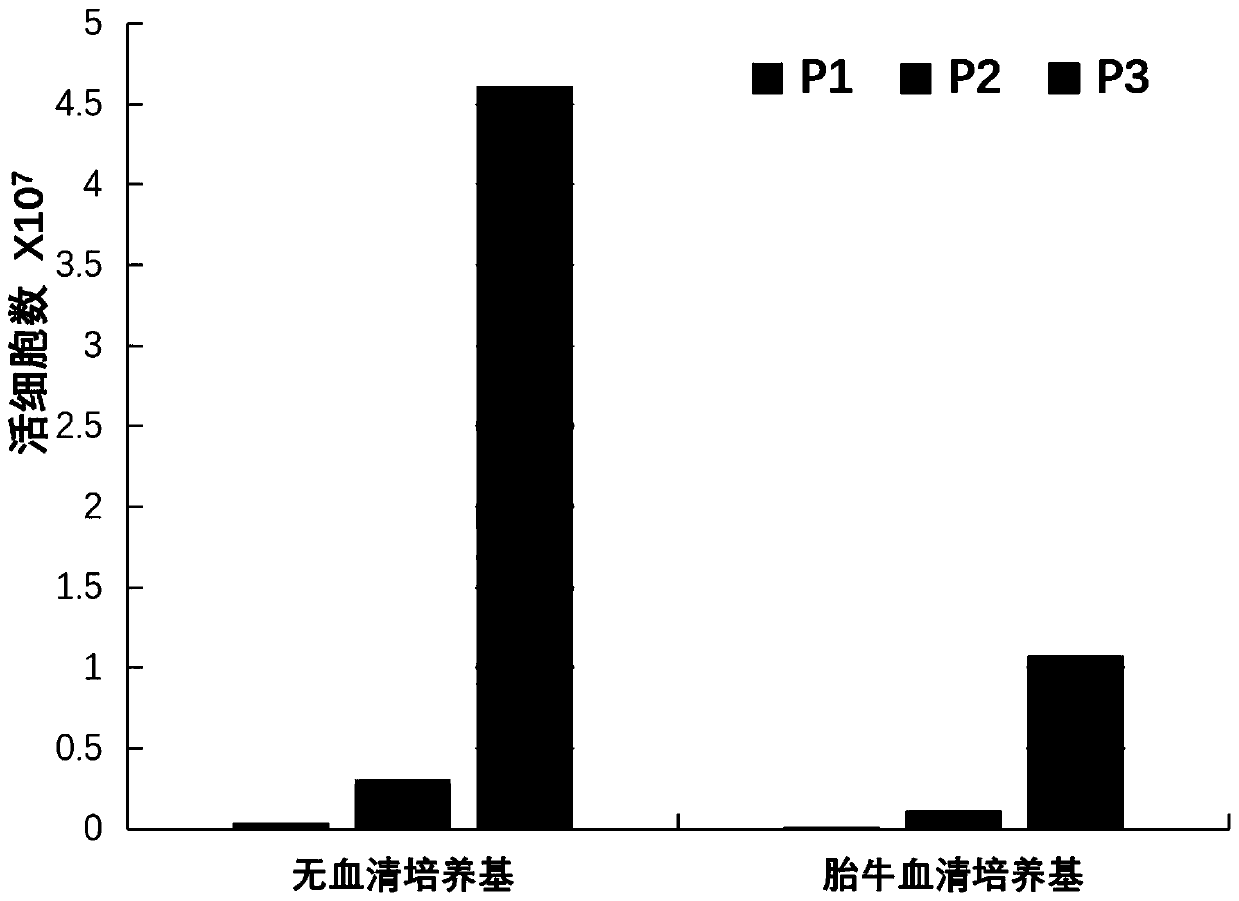
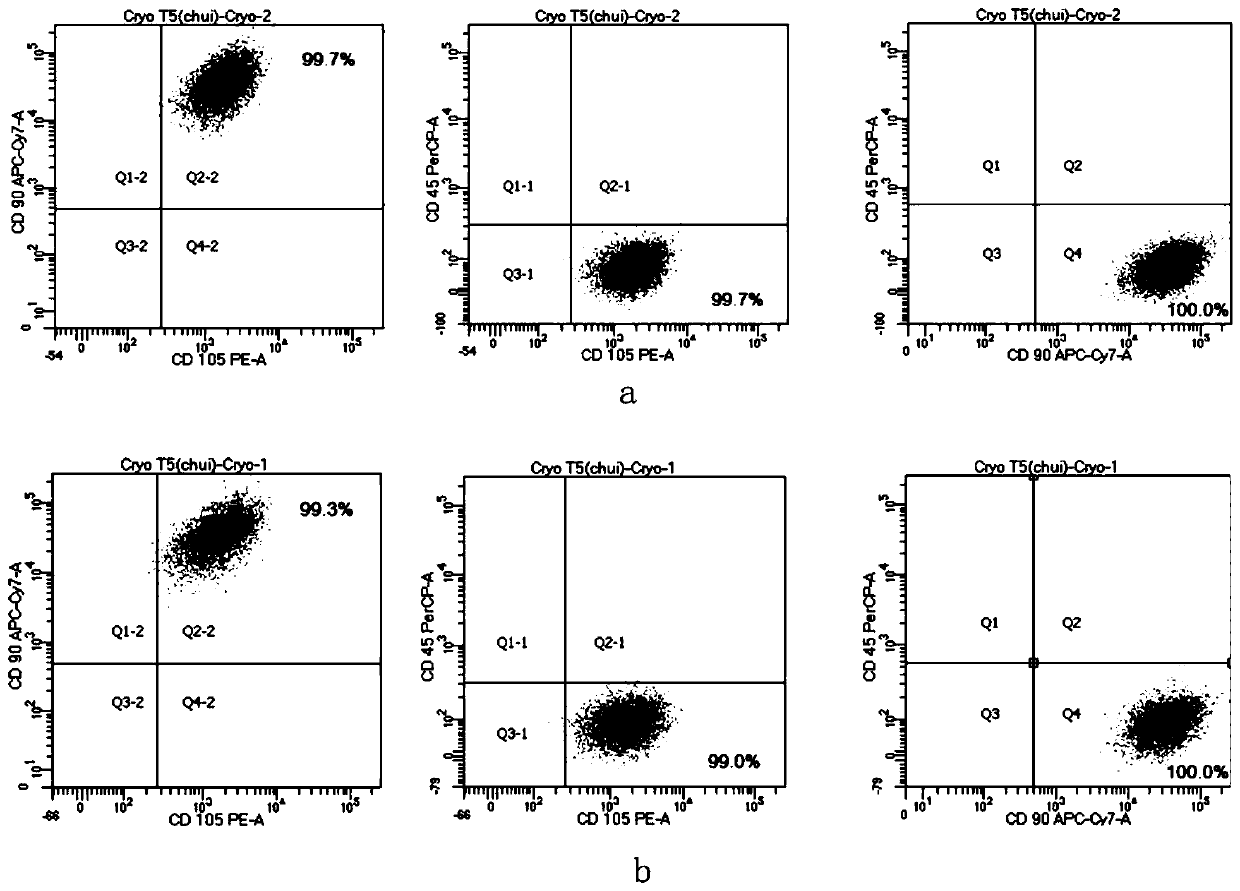
[0041] This example provides verification of the effect of the serum-free medium for mesenchymal stem cells in Example 1.
[0042] In the experiments of this example, human bone marrow mesenchymal stem cells were used. The cells were derived from the ATCC standard cell bank (PCS-500-012, Lot 70011720, ATCC). These cells were used in all the experiments below.
[0043] 1. Experimental method
[0044] 1. Cell culture
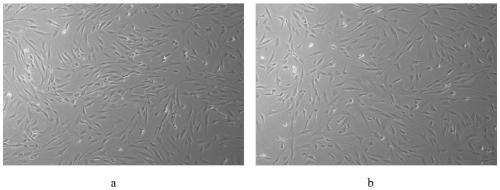
[0045] Human bone marrow mesenchymal stem cells, at 5000 / cm 2 The density was seeded on 100mm cell culture dishes. The cell culture dishes used in the experiment were all pre-coated and contained the following human adhesion molecules: 10 μg / cm 2 Human collagen IV (collagen IV), 5μg / cm 2 Human fibronectin.
[0046] Add mesenchymal stem cell serum-free medium and control medium containing bovine serum (DMEM / F12 medium (Sigma, M2906) with 10% fetal bovine serum (ExCellBio, FND500)) for continuous culture, the amount added is about 15ml , the medium was replaced...
Embodiment 3
[0061] This example provides verification of the effects of various culture media.
[0062] In the experiments of this example, human bone marrow mesenchymal stem cells were used. The cells were derived from the ATCC standard cell bank (PCS-500-012, Lot 70011720, ATCC). These cells were used in all the experiments below.
[0063] 1. Experimental method
[0064] 1. Preparation of culture medium:
[0065] serial number Medium solution volume ratio (DMEM / F12: MAX1) M1 10:0.4 M2 10:0.6 M3 10:0.8 M4 10:1.0 M5 10:1.6 M6 commercial serum-free medium I M7 commercial serum-free medium II M8 serum containing medium
[0066] Wherein: the culture medium in numbering M1~M5 is the culture medium of basic medium and MAX1 under different volume proportions, and the component and content thereof of basic medium and MAX1 are the same as embodiment 1;
[0067] Commercial serum-free medium I: imported brand MSC serum-free medium (Milten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com