Methods for preparing 5-Aza-2'-deoxycytidine and intermediate product thereof
A technology for deoxycytidine and azacytosine, which is applied in the field of preparing the new azacytidine deoxynucleoside and intermediates for the preparation of 5-aza-2′-deoxycytidine, which can solve the problem that is not suitable for industrial production Expensive problems such as development, raw materials, protective groups and catalysts, etc., to achieve the effect of good reproducibility, low content and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
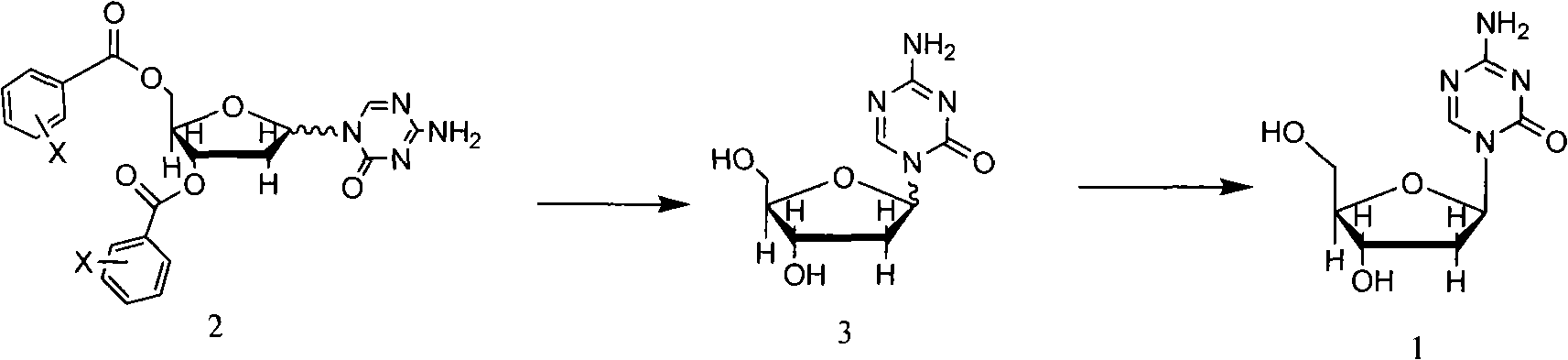
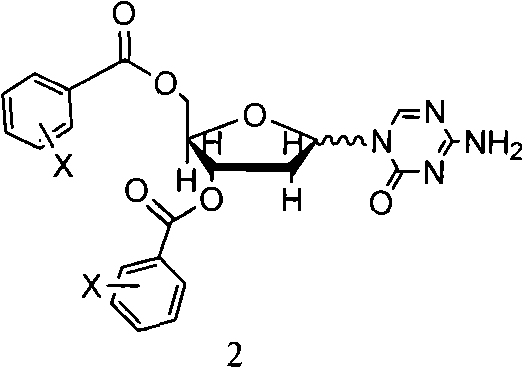
[0042] Preparation of 1-(2'-deoxy-3',5'-di-O-p-chlorobenzoyloxy-D-arabinofuranosyl)-5-azacytosine
[0043] Weigh 313.6 g of 5-azacytosine, put it in a reaction flask, add 8 L of hexamethyldisilazane, stir, add 200 ml of trimethylchlorosilane, protect it under nitrogen, heat to reflux, react for 24 hours, and dissolve the raw materials. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure by heating to obtain silanized 5-azacytosine, and the following reactions were carried out continuously without treatment.
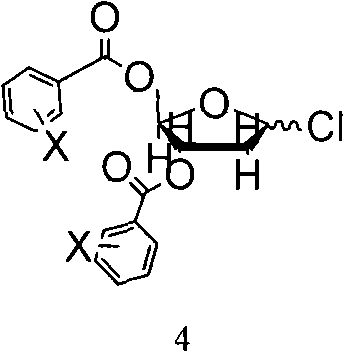
[0044] Add silanized 5-azacytosine to 16L of 1,2-dichloroethane, stir to dissolve, add 1-chloro-3,5-di-O-p-chlorobenzoyl-2-deoxy-D-ribose 395.6g (0.92mol), cooled to 5°C, temperature-controlled addition of a mixture of 333.6ml of trimethylsilyl trifluoromethanesulfonate (TMSOTf) and 2L of acetonitrile, and reacted at room temperature for 3.5h.
[0045] Pour the reaction solution into 30L of cold water, extract twice with dichlor...
Embodiment 2
[0052] Preparation of 5-aza-2'-deoxycytidine
[0053] Put 100L of methanol in a reaction flask, add 16 grams of sodium methoxide, stir, and add 1-(2'-deoxy-3',5'-di-O-p-chlorobenzoyloxy-D in Example 1 -Arabinofuranosyl)-5-azacytosine 510 grams, reacted at room temperature for 20 hours, concentrated to an appropriate volume, left to cool, filtered, washed with methanol, and dried in vacuo to obtain 150 grams of white crystal product, the related substances determined by HPLC were less than 1.5% .
[0054] Methanol was recrystallized again, as determined by HPLC, the related substance was 0.5%.
[0055] mp: 191.1-193.2;
[0056] [α] D 22 =+68.1° (30min), +57.8° (6hr) (c=0.5, H2O).
[0057] MS[M+H]+: 228.09;
[0058] Elemental analysis C8H12N4O4: theoretical C42.10, H5.30, N, 24.55, measured C42.02, H5.33, N, 24.35;
[0059] 1HNMR test (DMSO-d6) δ: 2.13~2.21(m, 2H), 3.54-3.61(m, 2H), 3.82(t, 1H), 4.24(m, 1H), 5.01(m, 2H), 5.20( d, 1H), 6.03 (t, 1H), 7.47 (s, 2H), 8.51 (s,...
Embodiment 3
[0062] Preparation of α-isomer of 2'-deoxy-5-azacytosine nucleoside
[0063] The mother liquor of 5-aza-2'-deoxycytidine prepared by the method of Reference Example 2 was subjected to column chromatography (gradient elution of chloroform-methanol system), and the fraction containing the α-isomer was collected and recrystallized from a mixed solvent of methanol / ether to obtain The alpha isomer of 5-aza-2'-deoxycytidine was used as white powder crystals.
[0064] 1HNMR (DMSO-d6): 8.26 (s, 1, H6), 7.4 (e, 2, NH2), 5.94 (d, 1, H1'), 5.21 (d, 1, OH3'), 4.83 (t, 1 , OH5'), 4.25(m, 2, H3', 4'), 3.4(m, 2, H5'), 2.3(m, 2, H-2');
[0065] Mp: Decompose at 181-182°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com