Process for the synthesis of azacitidine and decitabine
A technology of azacitidine and decitabine, applied in the field of synthesis of azacitidine-ketone) and decitabine-ketone), achieving the effect of high simplicity and avoiding the steps of handling and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
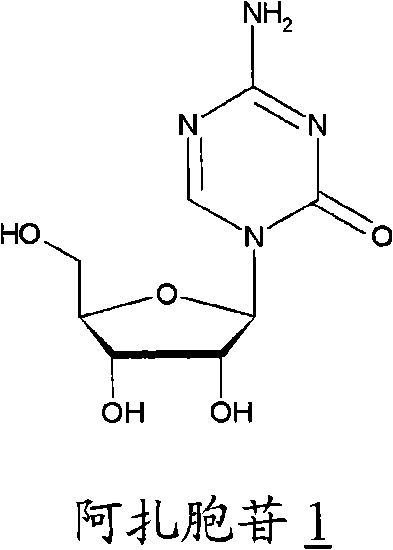
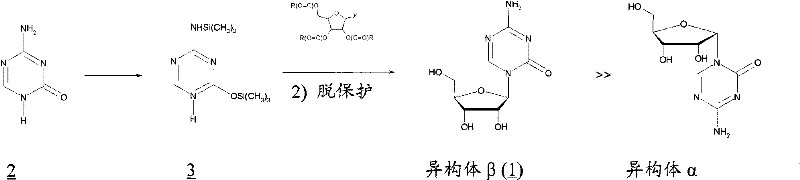
[0058] Example 1. Preparation of Azacitidine
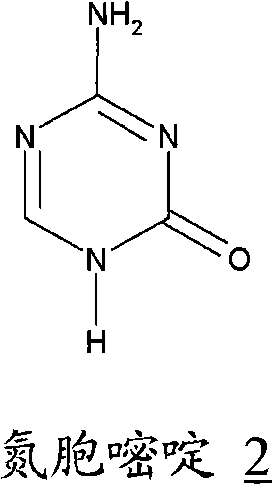
[0059] 34.7 mL of BSTFA was added to a suspension of 6.3 g of azacytosine in 126 mL of dichloromethane. The mixture was brought to reflux temperature and stirred for 90 minutes. In about 10 minutes, 11.35 mL of trimethylsilyl triflate was added to the clear solution, followed by 16.7 g of 1,2,3,5-tetra-O-acetyl-D-ribofuranose A solution in 33 mL of dichloromethane was added to the clear solution. Reflux and stirring are maintained for 2 hours, it is cooled to room temperature and it is poured into a solution of 9.5 g of sodium bicarbonate in 330 ml of water. It was stirred for 10 minutes and the pH was adjusted to pH=4.5 using acetic acid. Allowed to settle, the phases were separated and the aqueous phase was washed twice with 125 ml of dichloromethane per wash. The organic phases anhydrated by treating them with anhydrous calcium chloride were combined. Concentration was carried out under reduced pressure until a viscous re...
Embodiment 2
[0061] Example 2. Preparation of Azacitidine
[0062] 34.7 mL of BSTFA was added to a suspension of 6.3 g of azacytosine in 126 mL of acetonitrile. The mixture was brought to 50°C and stirred for 90 minutes. In about 10 minutes, 11.35 mL of trimethylsilyl triflate was added to the clear solution, followed by 16.7 g of 1,2,3,5-tetra-O-acetyl-D-ribofuranose A solution in 33 mL of acetonitrile was added to the clear solution. Stirring was maintained for 75 minutes, it was cooled to room temperature and it was poured slowly into a mixture of 500 ml of dichloromethane and 330 ml of water into which 9.5 g of sodium bicarbonate had previously been dissolved. Stirring was carried out for 15 minutes and the pH was adjusted to pH=4.5 using acetic acid. The mixture was allowed to settle and the phases were separated. The aqueous phase was washed twice with 125 ml of dichloromethane for each wash. The organic phases, which were dehydrated by treating them with anhydrous calcium chl...
Embodiment 3
[0064] Example 3. Preparation of Azacitidine
[0065]34.7 mL of BSTFA was added to a suspension of 6.3 g of azacytosine in 126 mL of dichloromethane. The mixture was brought to reflux temperature and stirred for 90 minutes. In about 10 minutes, 62 mL of 1M tin tetrachloride in dichloromethane was added to this clear solution, followed by 16.7 g of 1,2,3,5-tetra-O-acetyl-D-ribofuranose in 33 mL The solution in dichloromethane was added to the clear solution. Reflux and stirring are maintained for 75 minutes, it is cooled to room temperature and it is poured into a solution of 9.5 g of sodium bicarbonate in 330 ml of water. It was stirred for 10 minutes and the pH was adjusted to pH=4.5 using 30% aqueous sodium hydroxide solution. Allowed to settle, it was filtered on an earth filter and the phases were separated, the aqueous phase was washed twice with 125 ml of dichloromethane for each wash. The organic phases, which were dehydrated by treating them with anhydrous calciu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com