Stable preparation method of decitabine freeze-dry preparation
A technology for decitabine and freeze-dried preparations, which is applied in the field of preparation of decitabine freeze-dried preparations, can solve problems such as increasing the toxic and side effects of drugs, and achieve the effects of reducing toxic and side effects, avoiding a large amount of degradation, and slowing down the degradation speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
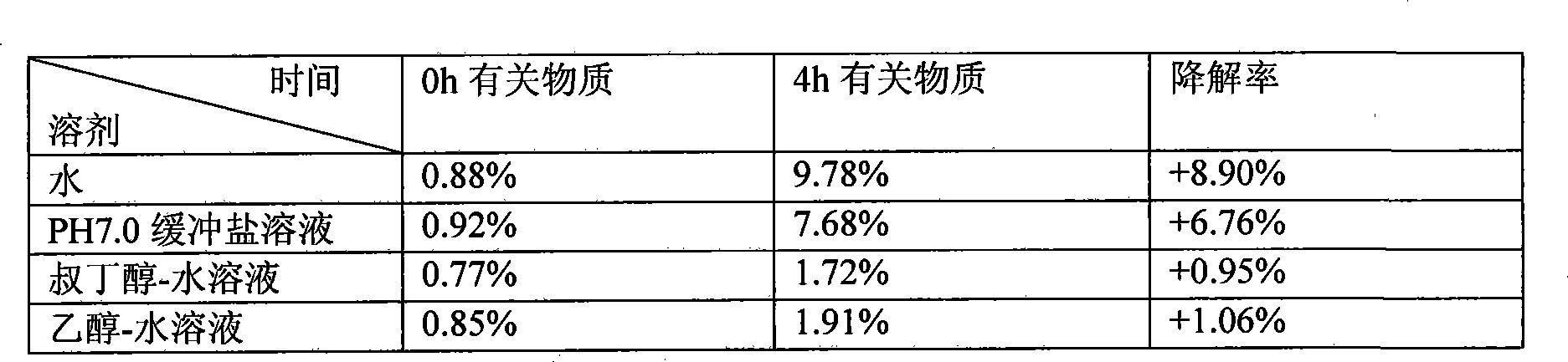
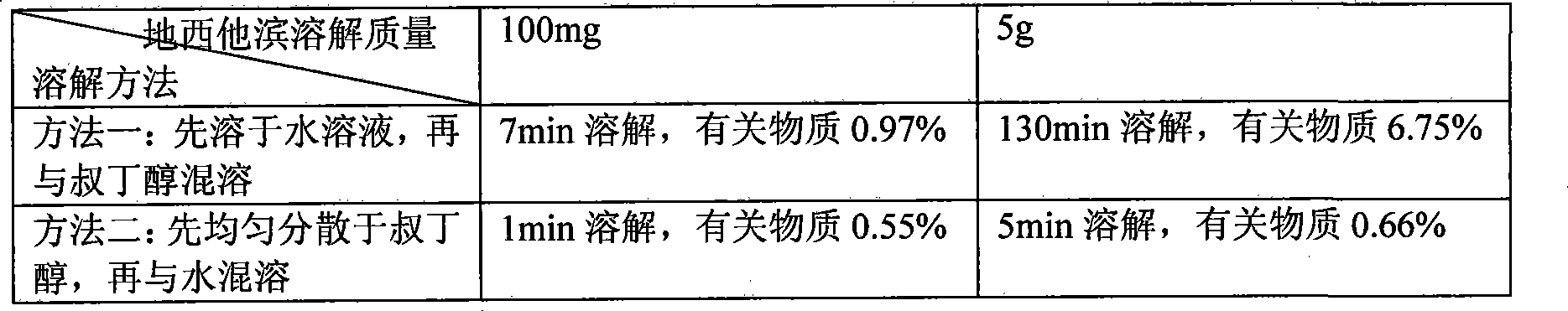
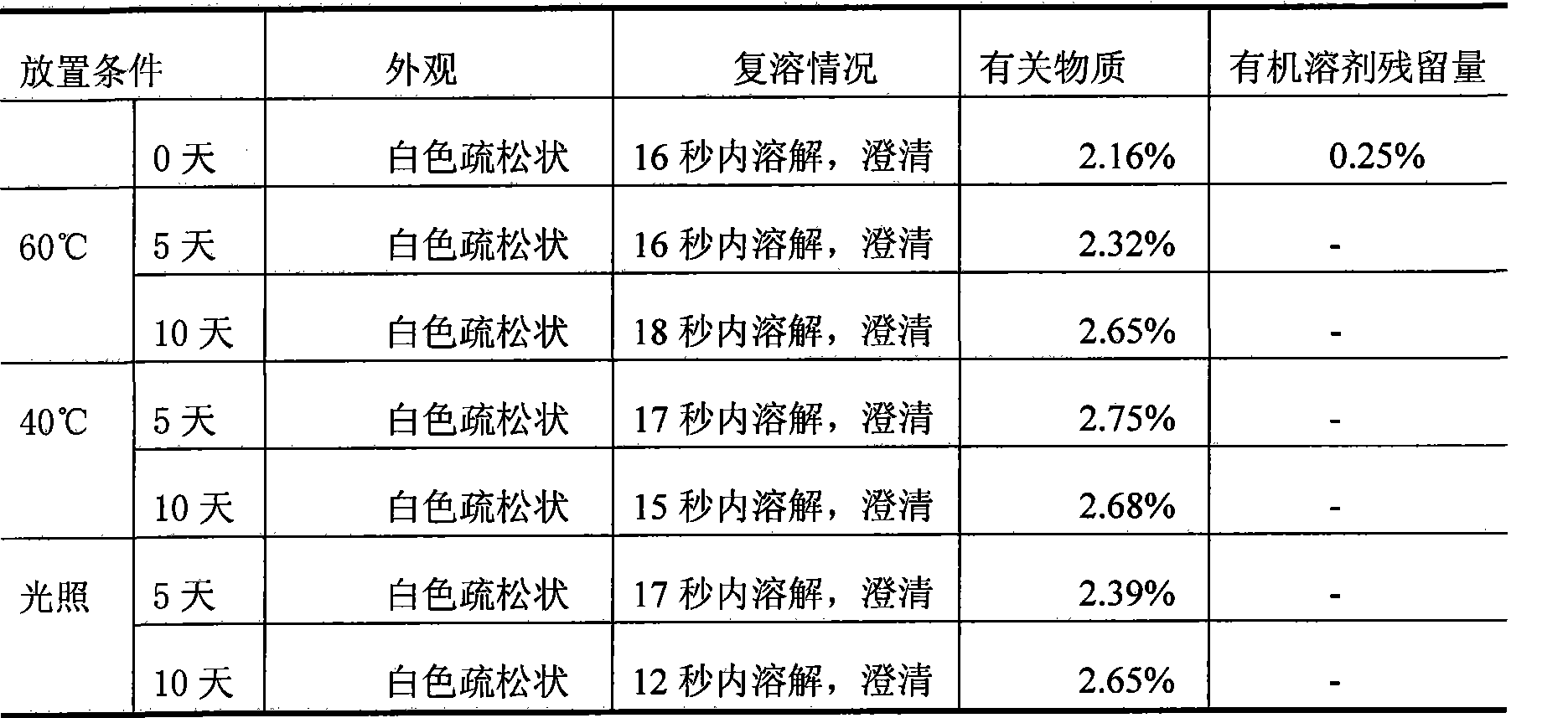
[0020] Disperse 50g of decitabine evenly in 6L of tert-butanol, add sodium hydroxide and sodium dihydrogen phosphate buffered saline solution with a pH of 6.5 to 7.5 to 10L for miscibility, and control the miscibility temperature at -10°C to obtain Citabine clear solution. 0.22um membrane filter, filled into vials and freeze-dried to remove tert-butanol-water mixture. Related substances of decitabine after dissolution ≤ 1%, almost no degradation occurs, after freeze-drying product decitabine related substances ≤ 3%, residual organic solvent is 0.31%, and is still stable through the test of influencing factors, related substances Still < 3%.
preparation Embodiment 2
[0022] Evenly disperse 60 g of decitabine in 5 L of ethanol, add water to 10 L for miscibility, and control the miscibility temperature at 0° C. to obtain a clear solution of decitabine. 0.22um membrane filter, filled into vials and freeze-dried to remove ethanol-water mixture. Related substances of decitabine after dissolution ≤ 1%, almost no degradation occurs, after freeze-drying, related substances of decitabine ≤ 3%, residual organic solvent is 0.12%, and it is still stable through the test of influencing factors, related substances Still < 3%.
preparation Embodiment 3
[0024] Disperse 70 g of decitabine evenly in 3 L of methanol, add a mixed solution of lactose and potassium dihydrogen phosphate with a pH of 5.5 to 6.5 to 10 L for miscibility, and control the miscibility temperature at 10° C. to obtain a clear solution of decitabine. 0.22um membrane filter, filled into vials and freeze-dried to remove tert-butanol-water mixture. The related substances of decitabine after dissolution are ≤1%, almost no degradation occurs, the related substances of decitabine after lyophilization are ≤3%, and the residual organic solvent is 0.09%, and it is still stable through the test of influencing factors. Still < 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com