Synthesis of decitabine
A decitabine, coupling reaction technology, applied in the direction of carbohydrate active ingredients, drug combinations, esterification saccharides, etc., can solve the problem of not developing and utilizing tin tetrachloride and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-3
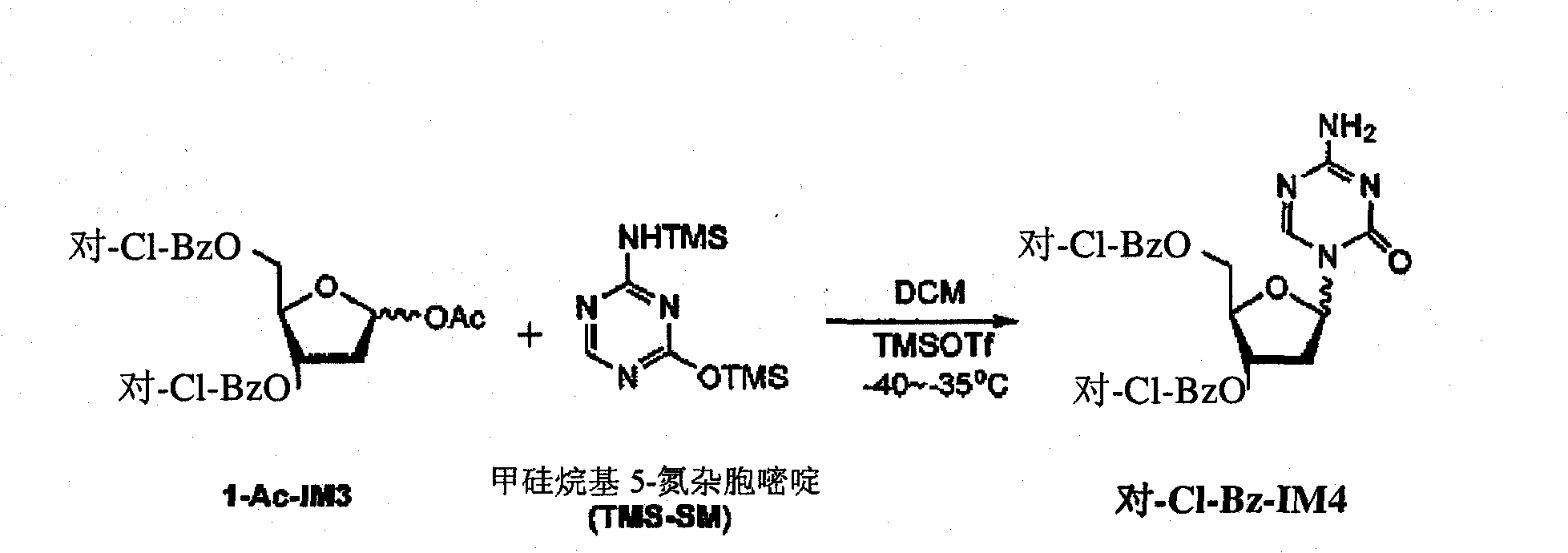
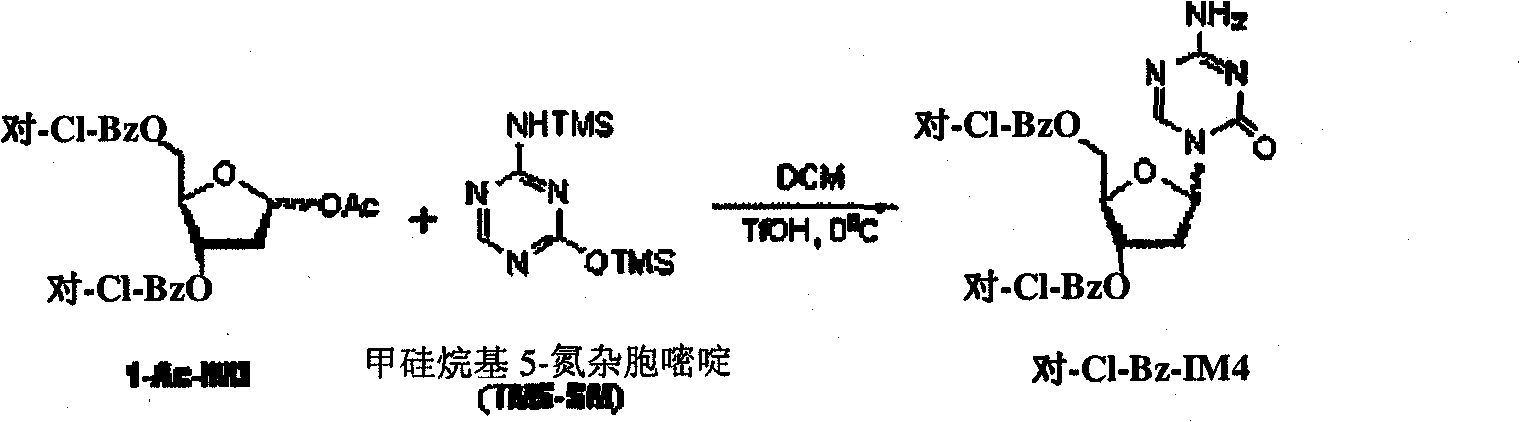
[0010] Example 1-3, the preparation of 5-two-O-(p-chlorobenzoyl)-decitabine
[0011]
[0012] 1-O-acetyl-3,5-di-O-(p-chlorobenzoyl)-2-deoxy-D-ribofuranose (500 g, 90% HPLC purity, equivalent to 0.99 mol), dichloromethane DCM (5.93 Kg) and silyl 5-azacytosine (254 g, 0.99 mol) were cooled to -45°C to -40°C and TMSOTf (231 g, 1.04 mol) was added and cooled at -40°C to -35°C The solution was stirred for 9h. 33% MeNH in MeOH at -40°C to -35°C 2 (97.6 g, 1.04 mol) was added to the reaction solution which was then diluted with DCM (5.93 Kg). Warm the solution to 20°C to 25°C and add NaHCO at 20°C to 25°C 3 The solution was saturated (8.3Kg) and it was stirred for 30min to 40min. separate the organic phase, Molecular sieve dried and filtered and rinsed with additional DCM (3.7Kg) Molecular sieve. The filtrates were combined and evaporated to dryness. The solid was dried in vacuo at 50°C, then ground to a fine powder and dried again in vacuo at 50°C. 460 g of the title c...
example 2
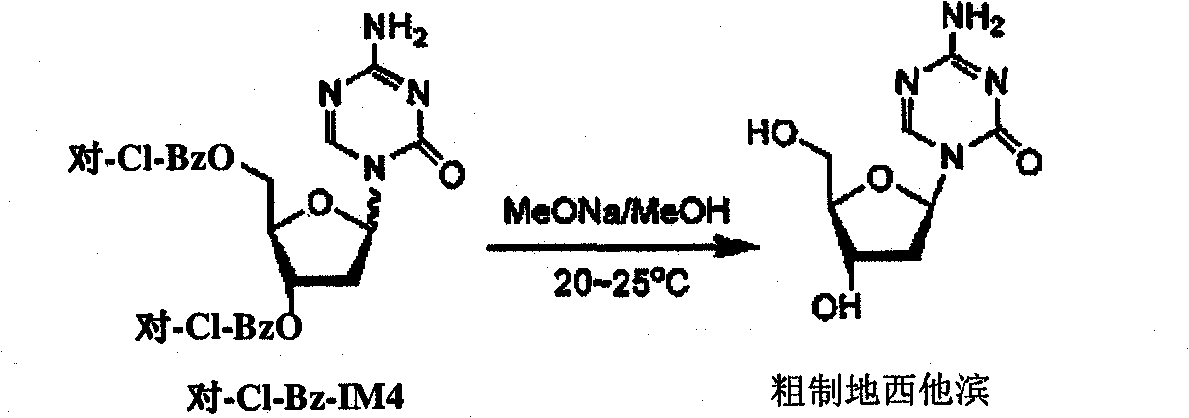
[0013] Example 2 - crude decitabine
[0014]
[0015] MeOH (1.8 Kg) and 3,5-di-O-(p-chlorobenzoyl)-decitabine (455 g, 63.9% HPLC purity, corresponding to 0.58 mol) were stirred at 20°C to 25°C. 29% MeONa in MeOH (42.8 g, 0.23 mol) was added to the mixture, which was then stirred at 20°C to 25°C for 30 min. The solid was filtered, washed 3 times with n-heptane (120 mL each), and then dried in vacuo at 50° C. to obtain 42.5 g of crude decitabine with a purity of 93.6% indicated by HPLC analysis.
example 3
[0016] Example 3 - Purification of crude decitabine with MeOH
[0017] Crude decitabine (42.5 g) and MeOH (2.85 kg) were stirred and heated to reflux until the mixture was almost completely dissolved. The solution was filtered hot to remove insoluble material. The filtrate was stirred and cooled, and crystals started to form at about 40°C. The resulting slurry was stirred at cloud point for 1 h, then cooled further slowly. The slurry was stirred at 10°C to 25°C for 4-8h, then filtered. The filter cake was washed 3 times with MeOH (40 mL each) and dried in vacuo at 50 °C for 8 h to obtain 27.5 g of pure decitabine with a yield of 64.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com