Preparation method of decitabine intermediate
A technology for decitabine and intermediates, applied in the preparation of sugar derivatives, chemical instruments and methods, esterified saccharides, etc., can solve the problems of high cost, low market supply, harsh reaction conditions, etc., and achieve easy operation and control , reduce operating hazards, and obtain raw materials easily
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
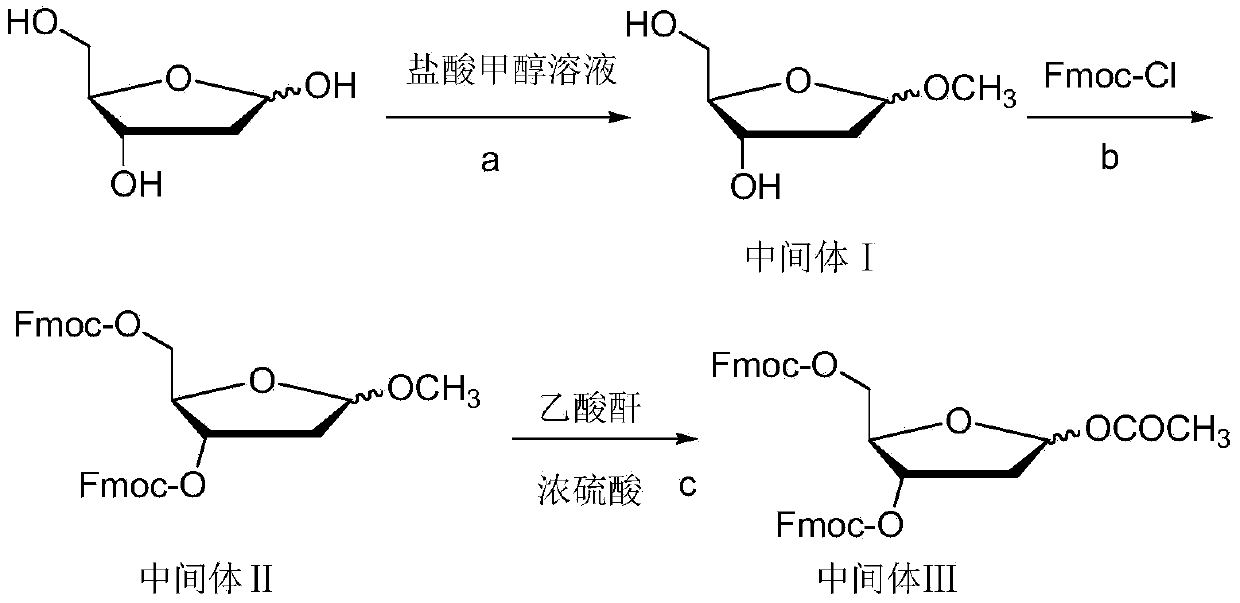
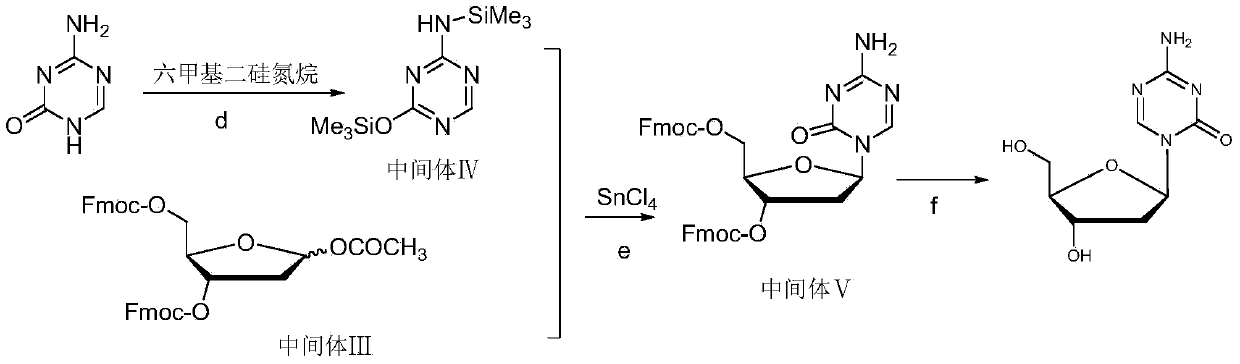
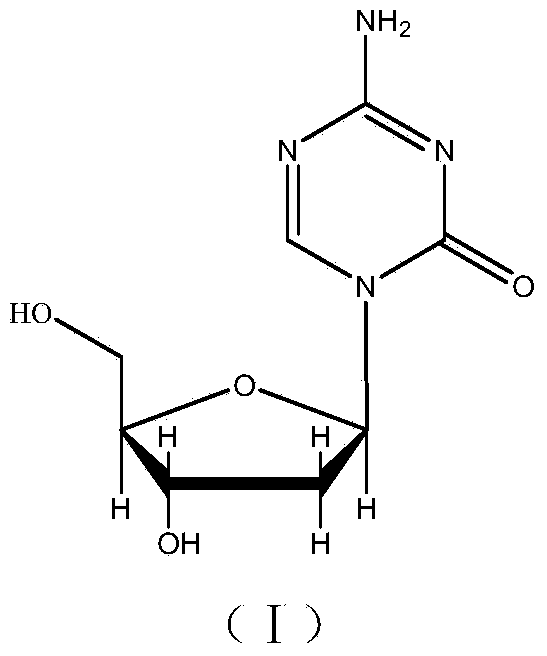
[0067] Synthesis of Intermediate I
[0068] Add 1200g of 2-deoxy-D-ribose and 12L of anhydrous methanol into the reaction kettle, and stir at room temperature until the solid is completely dissolved, and the system is a light yellow transparent solution. 2.4 L of HCl-methanol solution with a mass fraction of HCl of 1% was added dropwise through a constant pressure funnel. After the dropwise addition was complete, it was stirred for 40 minutes. 600 ml of pyridine was added and stirring was continued for 30 minutes. After suction filtration, the filter cake was washed with anhydrous methanol, and the filtrate was evaporated to dryness under reduced pressure at 0.01Mpa to obtain 2.1Kg of intermediate I as an oil.
[0069] Synthesis of Intermediate II
[0070] Add 1.185Kg of intermediate I and 8L of anhydrous pyridine into the reaction kettle, control the reaction temperature at -20°C, add 4.552KgFmoc-Cl in three batches, each with an interval of 1 hour, after the addition is c...
Embodiment 2
[0074] Synthesis of Intermediate I
[0075] Add 1200g of 2-deoxy-D-ribose and 12L of anhydrous methanol into the reaction kettle, stir at room temperature until the solids are completely dissolved, and the system is a light yellow transparent solution. 2.4 L of HCl-methanol solution with a mass fraction of HCl of 0.5% was added dropwise through a constant pressure funnel. After the dropwise addition was complete, it was stirred for 40 minutes. 600 ml of pyridine was added and stirring was continued for 30 minutes. After suction filtration, the filter cake was washed with anhydrous methanol, and the filtrate was evaporated to dryness under reduced pressure at 0.01Mpa to obtain Intermediate I as an oil.
[0076] Synthesis of Intermediate II
[0077] Add 1.185Kg of intermediate I, 8L of anhydrous pyridine to the reaction kettle, control the reaction temperature between 0°C, add 4.552KgFmoc-Cl in two equal batches, and the interval between the two batches is half an hour. After...
Embodiment 3
[0081] Synthesis of Intermediate I
[0082] Add 1200g of 2-deoxy-D-ribose and 12L of anhydrous methanol into the reaction kettle, and stir at room temperature until the solid is completely dissolved, and the system is a light yellow transparent solution. 2.4 L of HCl-methanol solution with a mass fraction of HCl of 1.2% was added dropwise through a constant pressure funnel. After the dropwise addition was complete, it was stirred for 40 minutes. 600 ml of pyridine was added and stirring was continued for 30 minutes. After suction filtration, the filter cake was washed with anhydrous methanol, and the filtrate was evaporated to dryness under reduced pressure at 0.01Mpa to obtain 2.1Kg of intermediate I as an oil.
[0083] Synthesis of Intermediate II
[0084] Add 1.185Kg of intermediate I, 8L of anhydrous pyridine to the reaction kettle, control the reaction temperature between 10°C, add 4.552KgFmoc-Cl in two equal batches, and the interval between the two batches is half an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com