Method for preparing improved decitabine
A technology of decitabine and its compound, which is applied in the field of preparation of decitabine, an anti-tumor cell growth drug, can solve the problems of low yield of deprotection group, difficult removal of heavy metal tin, excessive heavy metal and burning residue, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
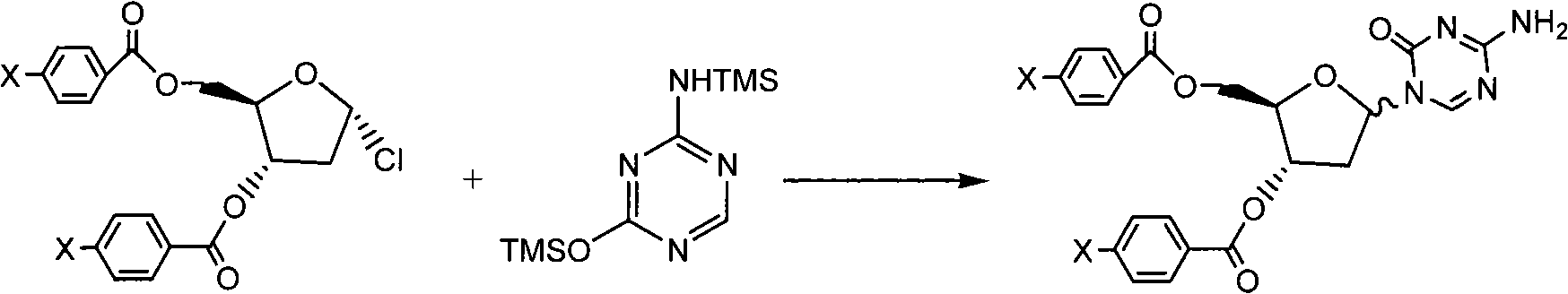
[0021] Preparation of 3',5'-di-p-chlorobenzoyl-5-aza-2'-deoxycytidine (Formula IV, X=Cl)
[0022] In the three-necked bottle of 1000ml, add 5-azacytosine (131 grams, 1.17mol), hexamethyldisilazane (731ml, 3.51mol), ammonium sulfate (2g), reflux under nitrogen for 5 hours, reduce The solvent was distilled off under pressure to obtain 2,4-bis-(trimethylsilyl)-5-azacytosine (Formula III). Formula III was then dissolved in chloroform (2.4 L).
[0023] Add 1-α-chloro-3,5-di-p-chlorobenzoyl-2-deoxy-D-ribose (Formula II, X=Cl) (480g, 1.12mol) to the above solution, nitrogen protection, 50-55 Reflux for 4 hours. The reaction solution was cooled to room temperature, and the solvent was evaporated to dryness under reduced pressure. Methyl tert-butyl ether (2.4 L) was added to the residue, followed by heating under reflux for 1 hour. Cool to room temperature, filter with suction, and air-dry at 45°C to obtain an off-white solid, which is the title compound (Formula IV) (423.0 g), wit...
Embodiment 2-6
[0028] Preparation of 3′,5′-di-p-halobenzoyl-5-aza-2′-deoxycytidine (Formula IV, X=Cl, Br) was performed in the same manner as in Example 1, and the results are shown in Table 1.
[0029] Table 1: Preparation of 3', 5'-di-p-halobenzoyl-5-aza-2'-deoxycytidine (Formula IV, X=Cl, Br)
[0030] Example
Embodiment 7
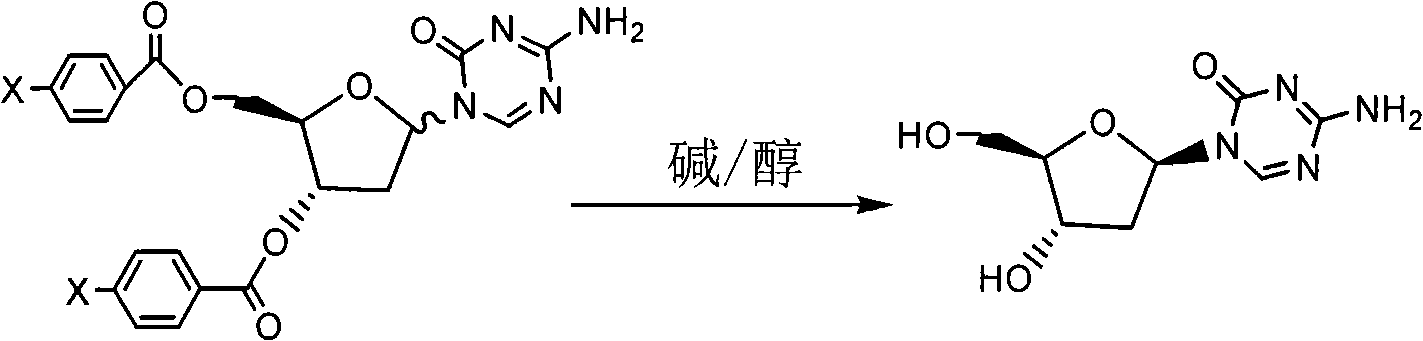
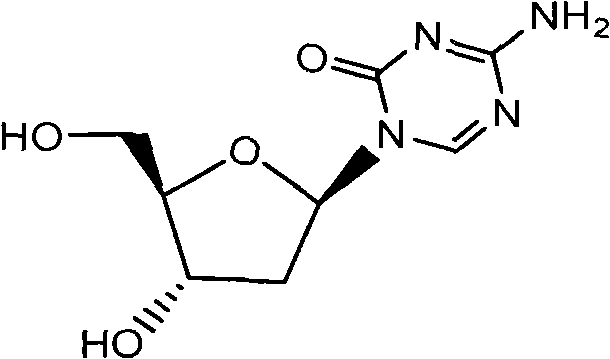
[0032] Preparation of Decitabine (Formula I)
[0033] 3', 5'-chlorobenzoyl-5-aza-2'-deoxycytidine (Formula IV, X=Cl) (200g, 396mmol) was added to methanol (2000ml), and sodium methoxide (1.07g, 19.8 mmol), refluxed under nitrogen for 3 hours. Cool to 0°C and stir for 2 hours, filter with suction, wash with cold methanol (200ml), and dry in vacuo to obtain white solid formula I (47.1g), yield 52%.
[0034] Melting point: 190-192°C; [α] 22 D +69.5°(C=1,H 2 O); purity 99.77%
[0035] 1 H-NMR (DMSO-d 6 )8.50(s, 1H), 7.46(bs, 2H), 6.01(t, 1H), 5.20(bs, 1H), 5.00(bs, 1H), 4.22(s, 1H), 3.80(bs, 1H), 3.51-3.61(m, 2H), 2.10-2.21(m, 2H)
[0036] Liquid Chromatography Conditions
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com