Patents
Literature
93 results about "Pyrimidine analogue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
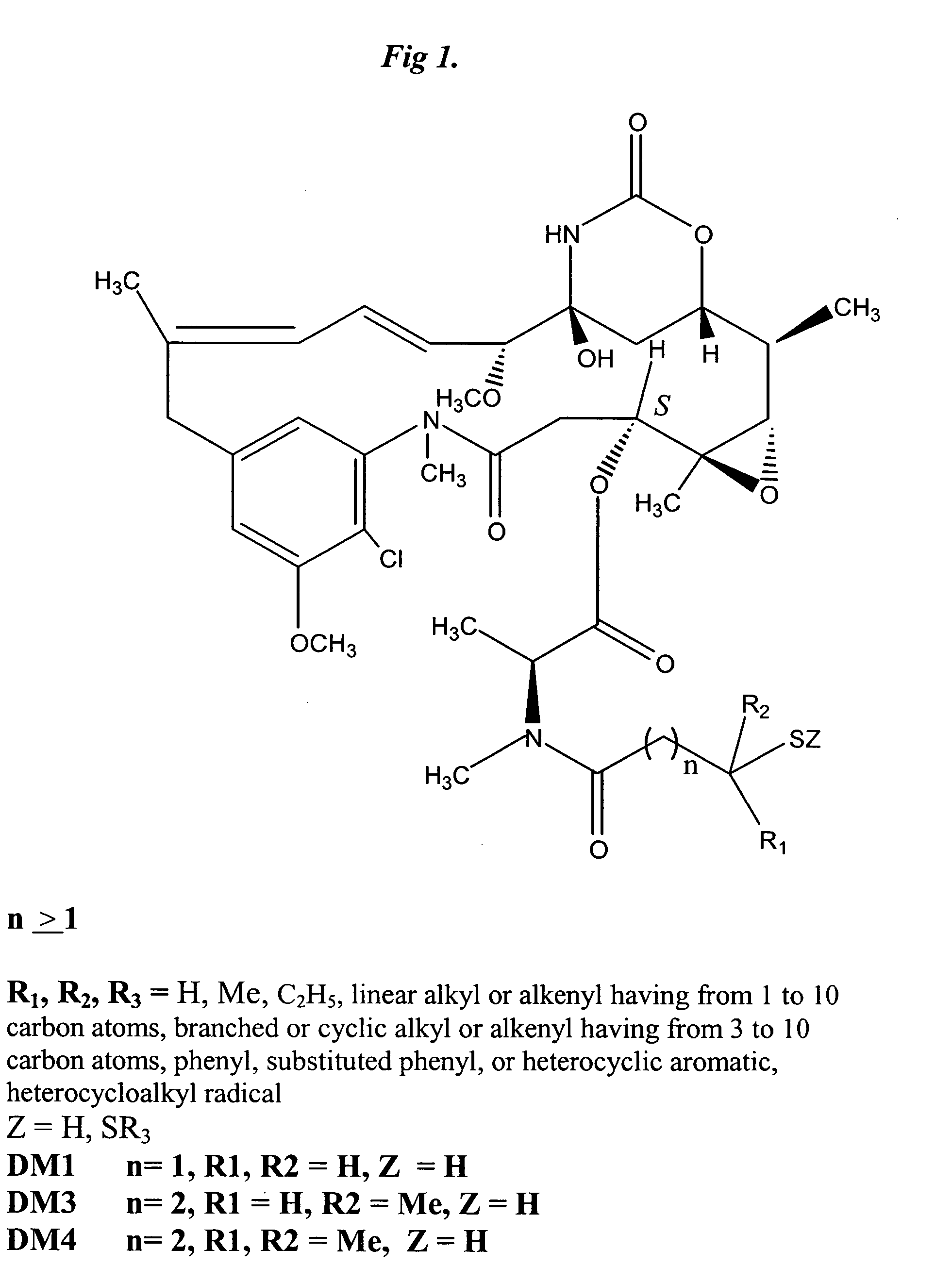
Pyrimidine analogues are nucleoside analog antimetabolites which mimic the structure of metabolic pyrimidines.
Oligonucleotides including pyrazolo[3,4-D]pyrimidine bases, bound in double stranded nucleic acids
A triplex forming oligonucleotide is complementary pursuant to the G / T or A / G recognition motif to a homopurine, or substantially homopurine target sequence in double stranded nucleic acids, and at least one and preferably all of the guanine bases are replaced by their pyrazolo[3,4-d]pyrimidine analog, namely by 6-amino-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one. The oliginucleotides containing the pyrazolo[3,4-d]pyrimidine analog of guanine exhibit a lesser degree of self-association, and lack the nucleophilic nitrogen atom in the 7 position of guanine. The latter feature results in a diminished extent of self-crosslinking in ODNs which also have a covalently attached cross-linking agent.
Owner:EPOCH PHARMA
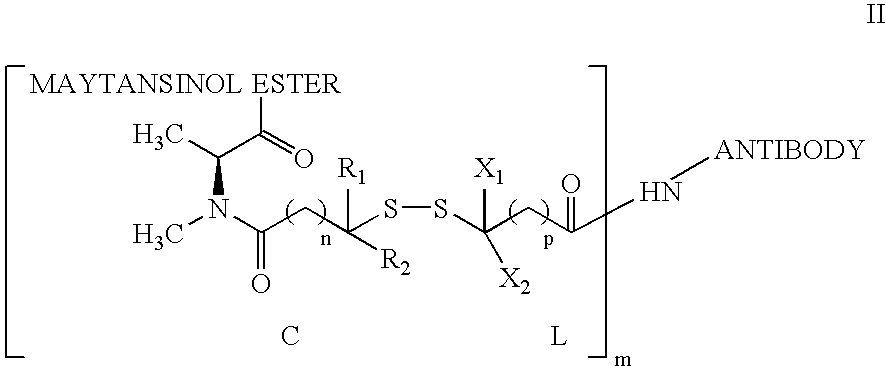
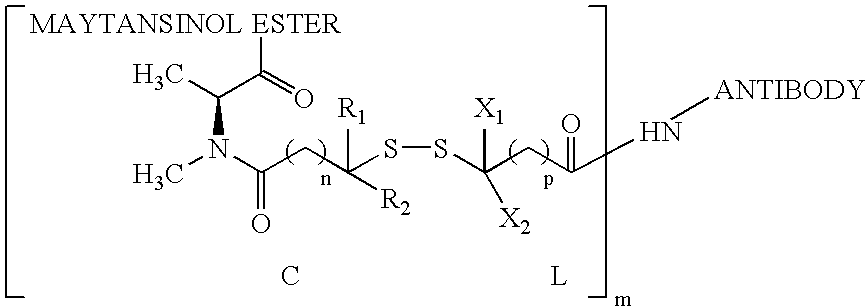
Anti-integrin immunoconjugates, methods and uses
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
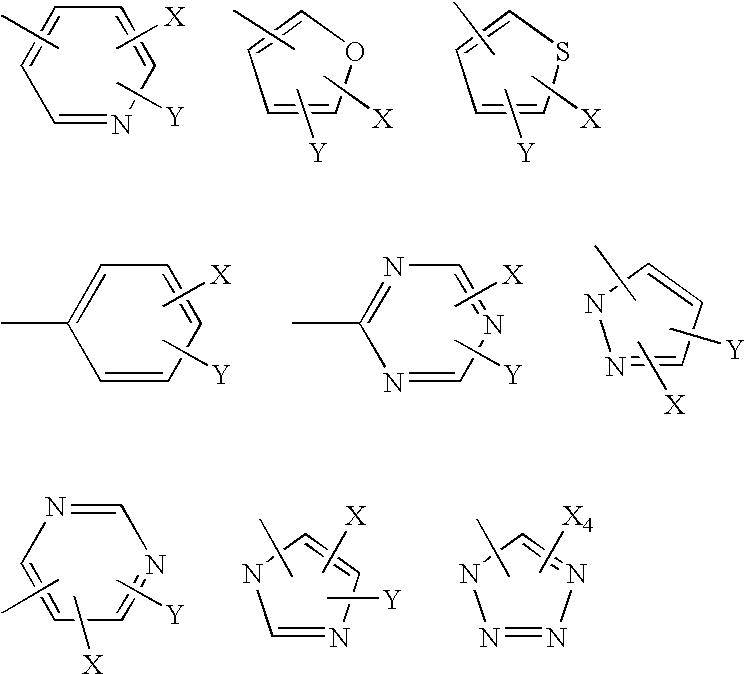
Aromatic heterocyclic pyridine derivatives and analogs and preparation method and application thereof
The invention aims at providing chemical synthesis and preparation of an aromatic heterocyclic pyridine and the analogs thereof to obtain derivatives and analogs of a plurality of series of aromatic heterocyclic pyridine and medicinal salts or salts having the following formulas or prodrugs, and the preparation, a pharmacologically activity experimental method and pharmacologically activity thereof are provided. The definitions of dotted line, ring A, ring B, X1, X2, X3, X4, R1, R2 and R3 in formula I are shown in the description. The invention provides the aromatic heterocyclic pyridine derivatives and analogue with antibacterial and antifungal activities, also provides the application thereof as antibacterial and antifungal drugs and the application thereof in concomitant use with other known antibacterial and antifungal drugs and with drugs for curing bacteria infection coupled with various complicating diseases such as inflammation, virus, immune system diseases and the like, and also provides the preparation method of the aromatic heterocyclic pyridine analogs.
Owner:LIAONING LIFENG SCI & TECH DEV
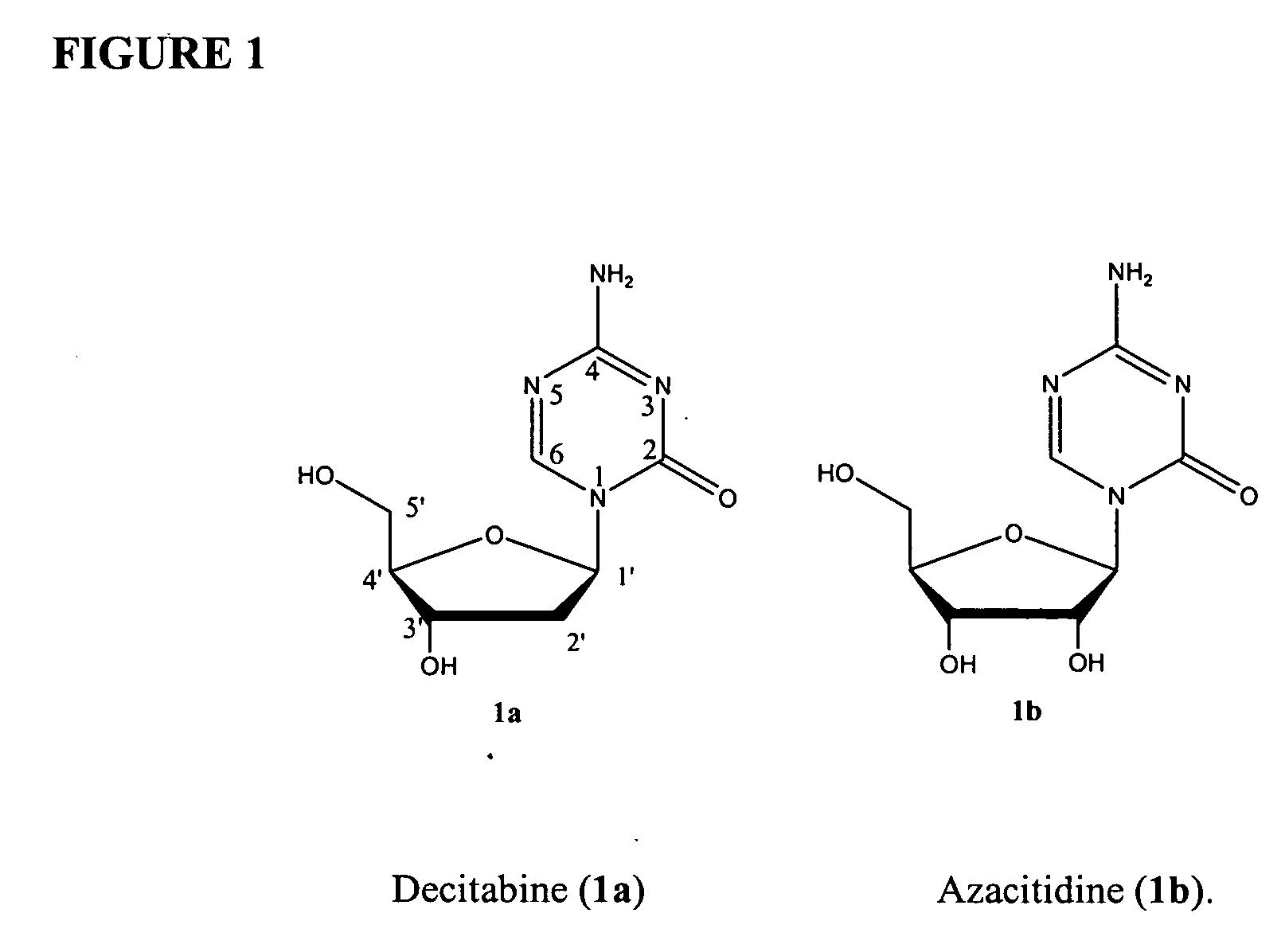
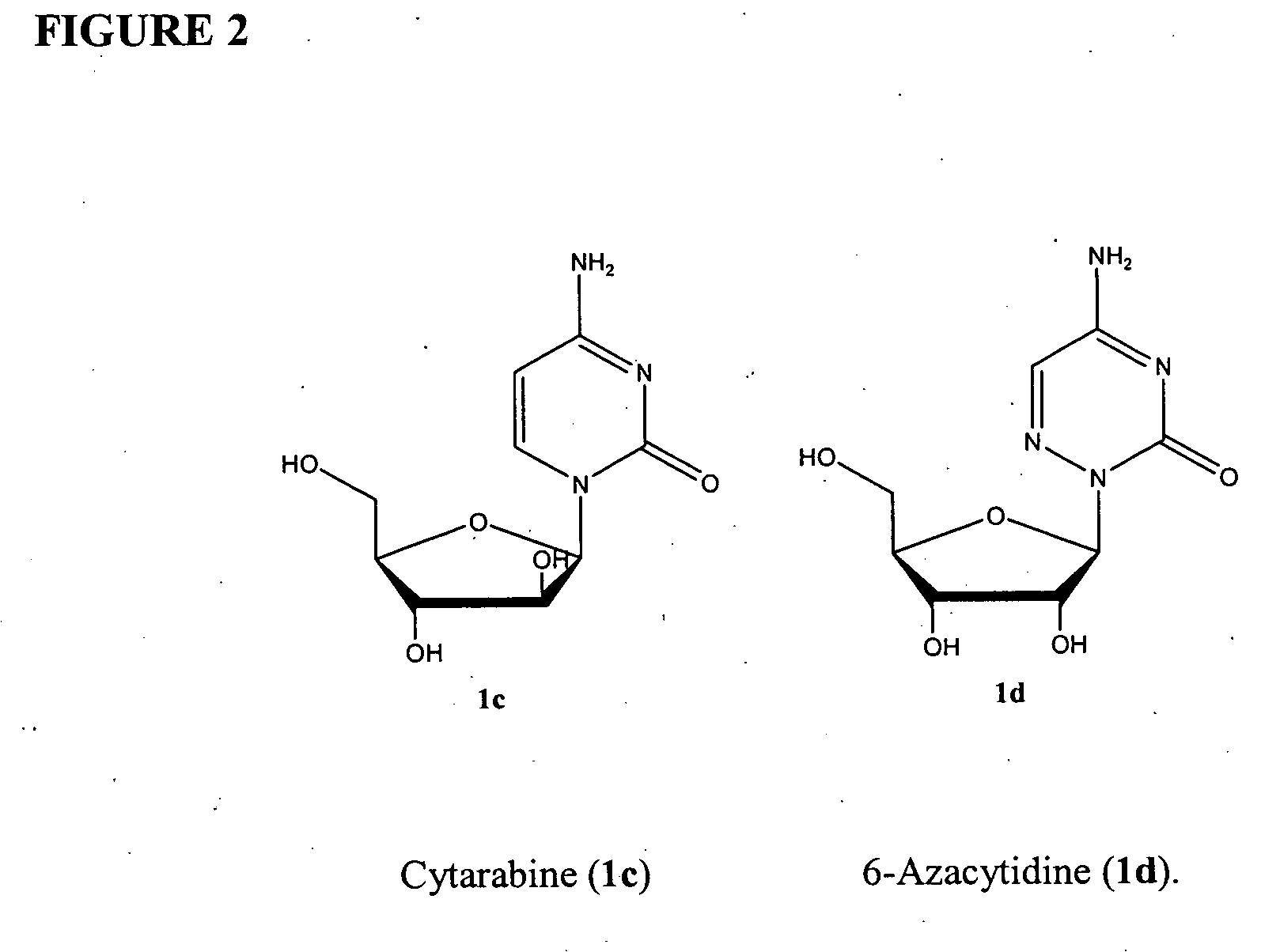
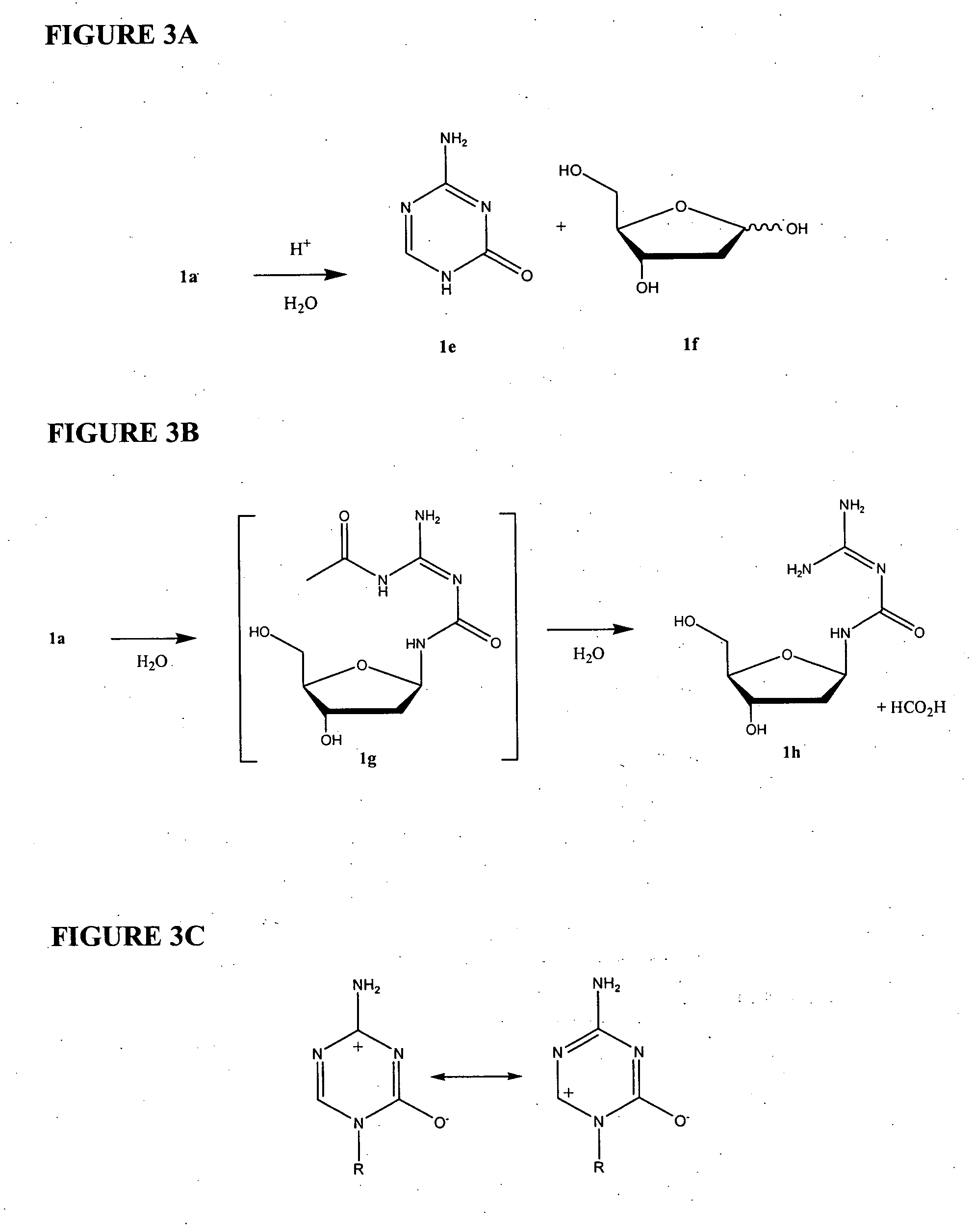
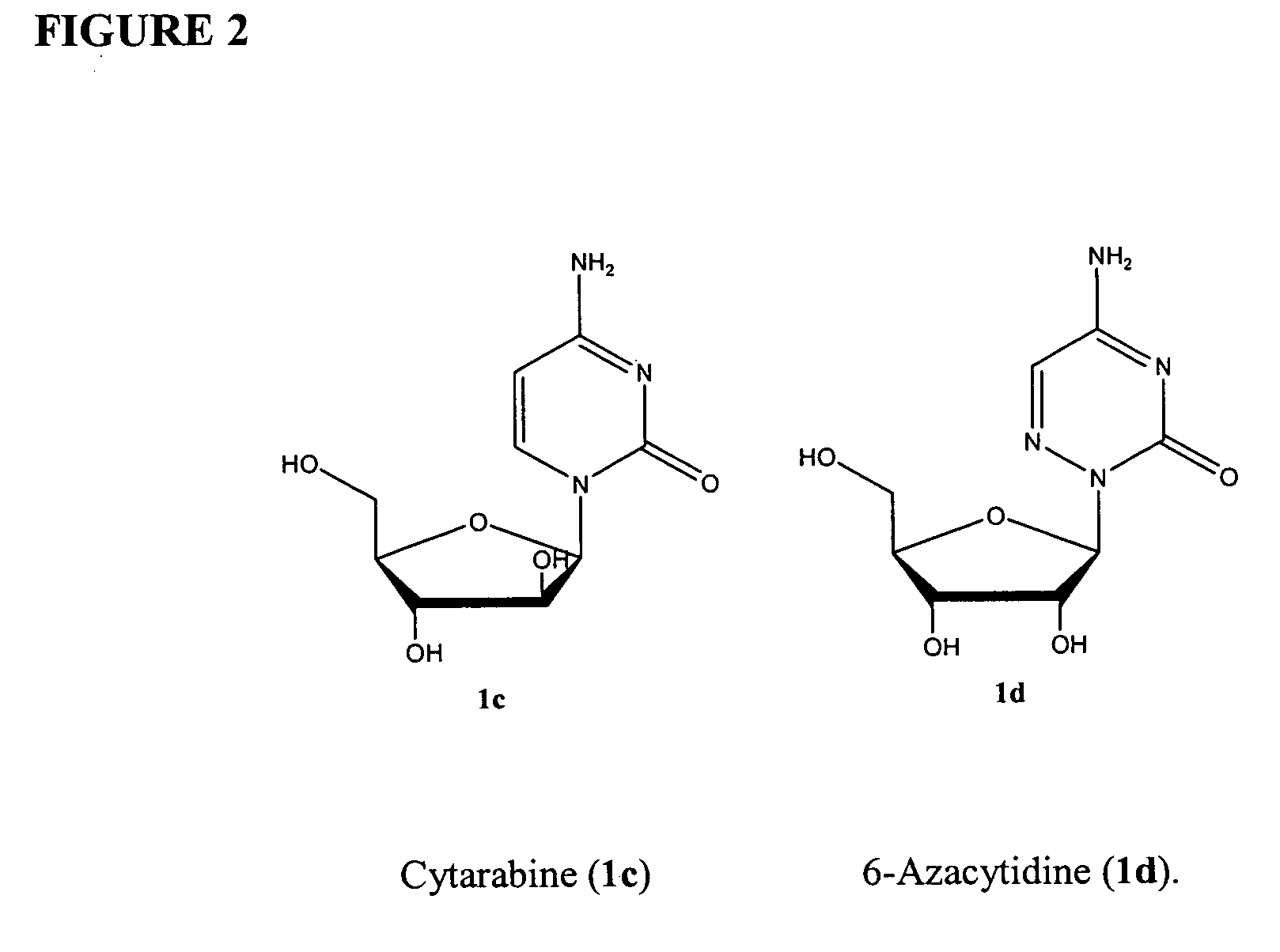
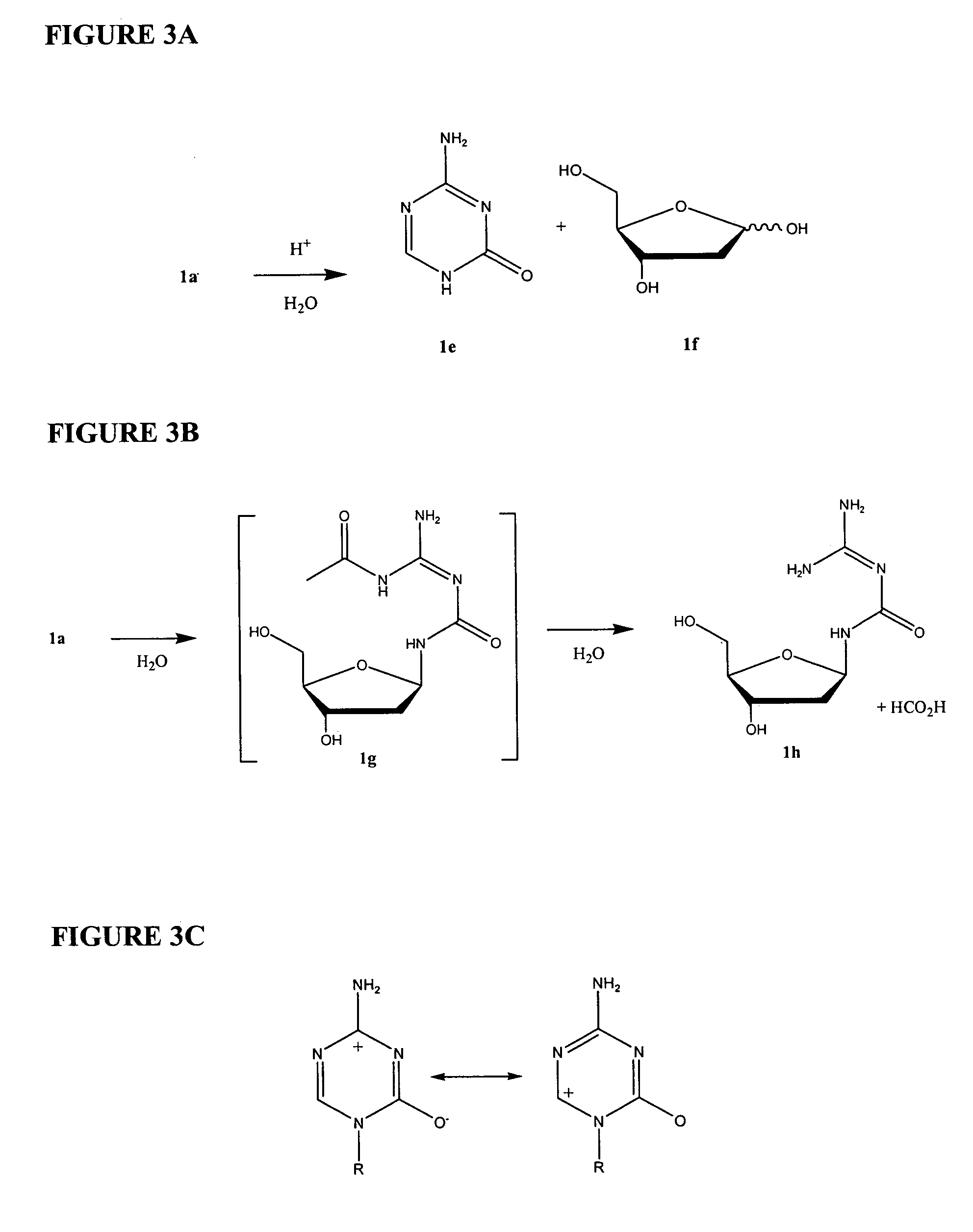
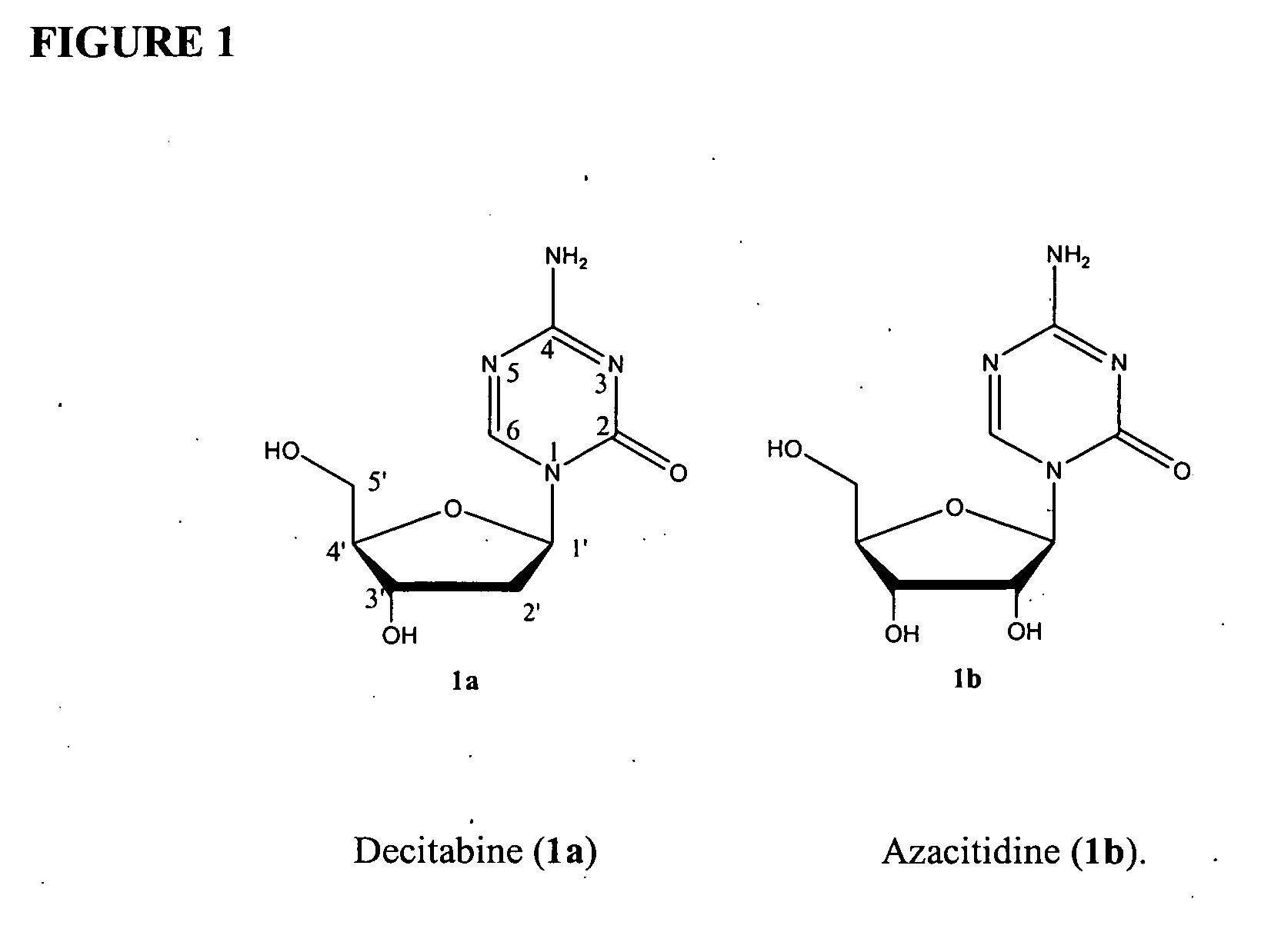
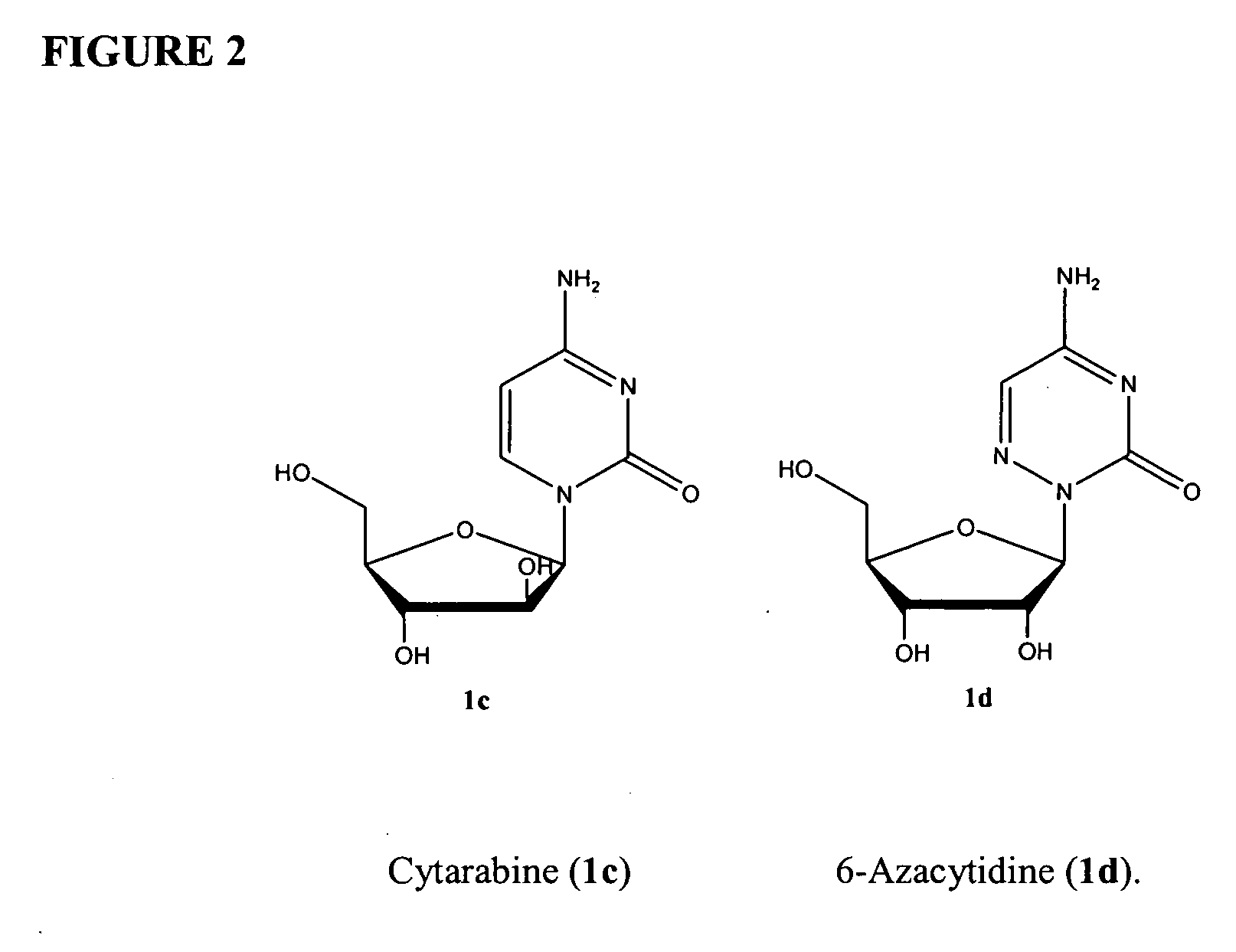
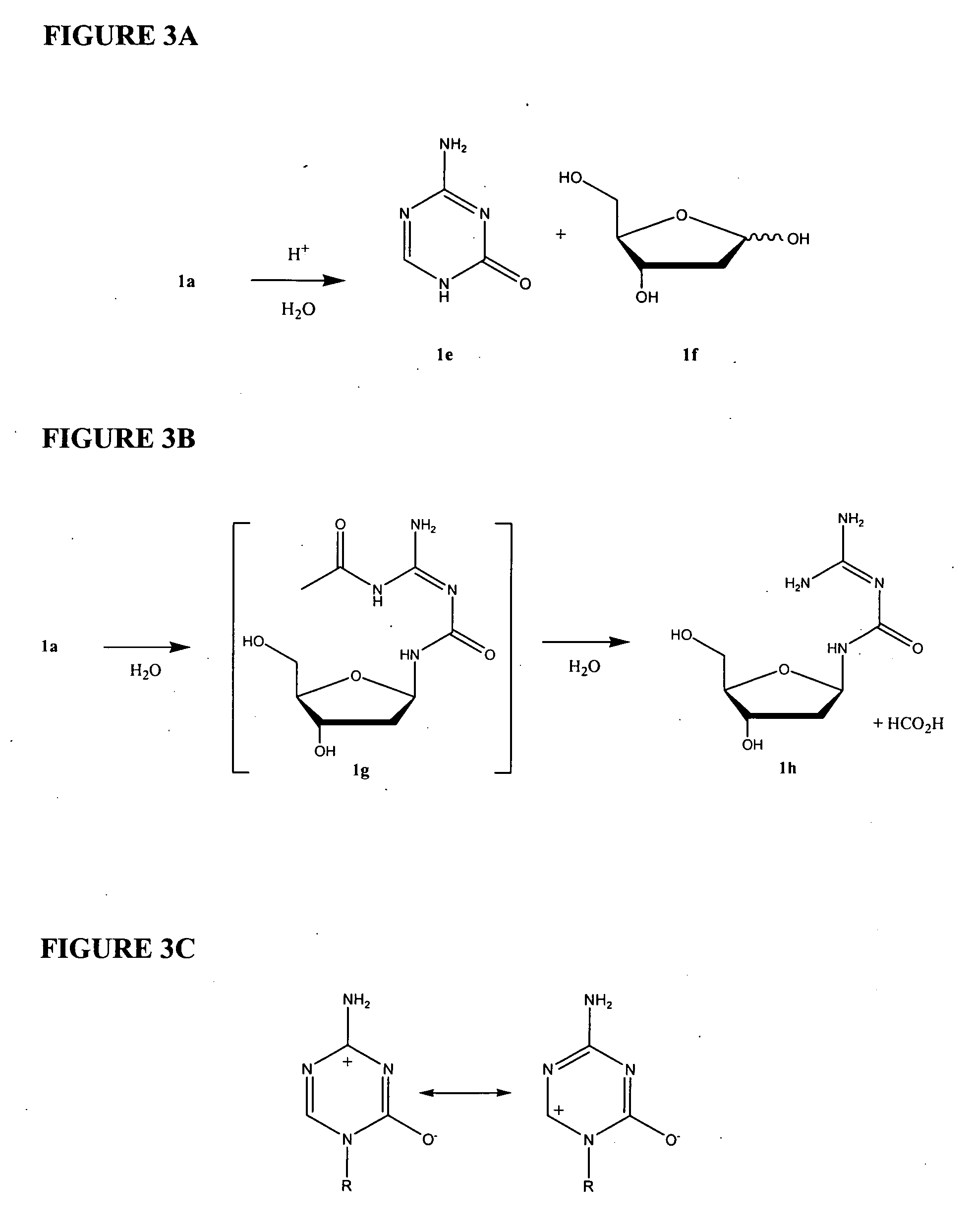
Azacytosine analogs and derivatives
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
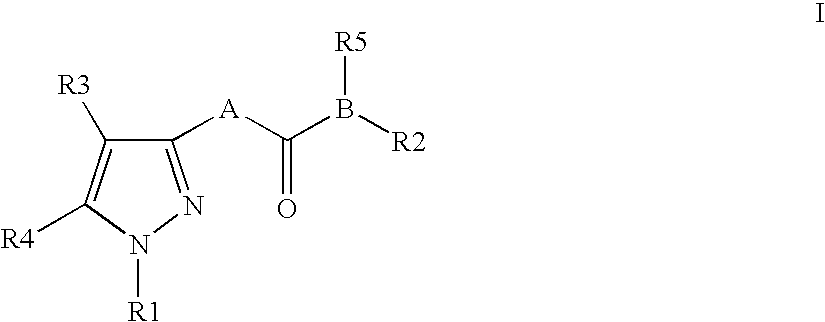
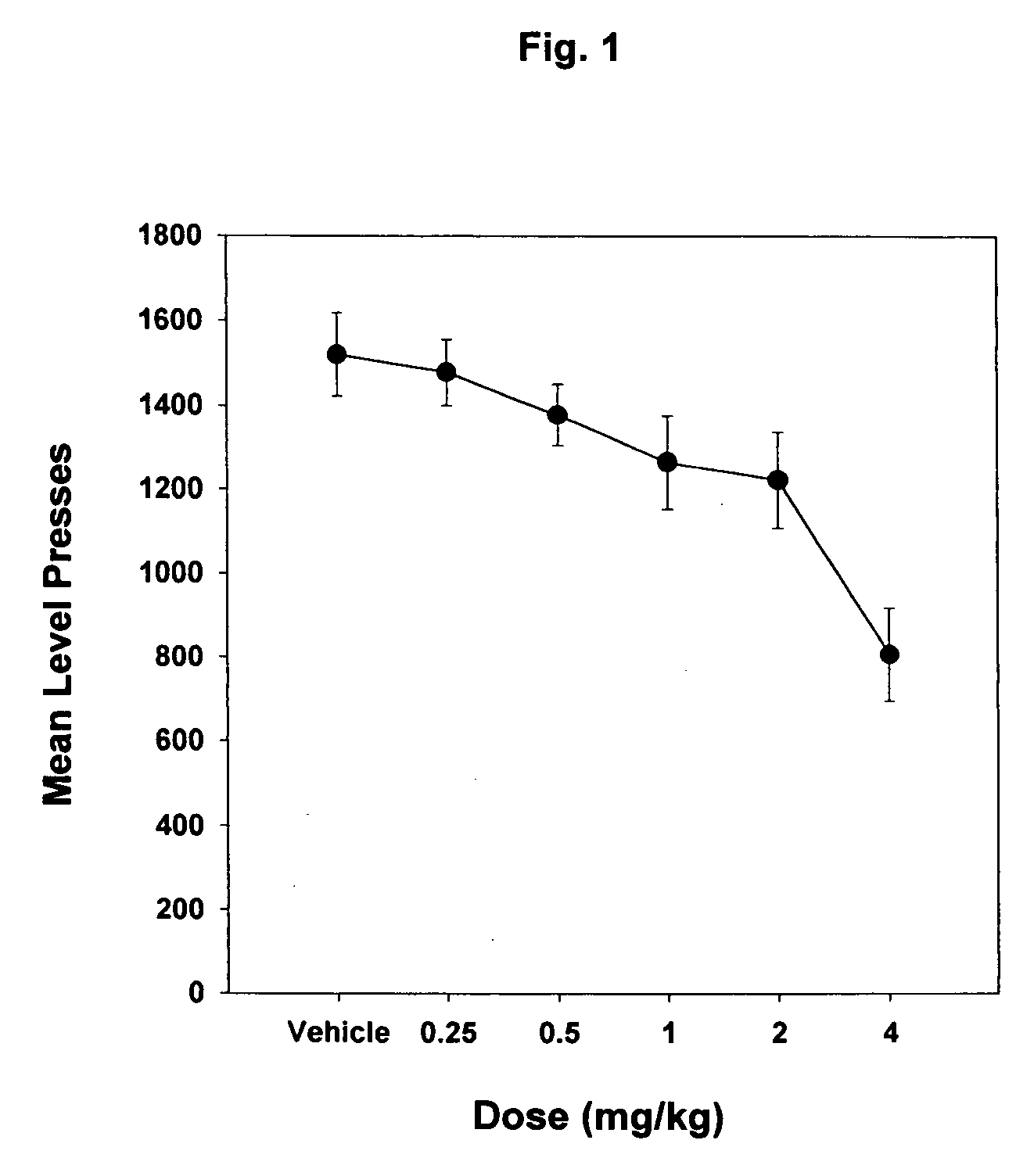
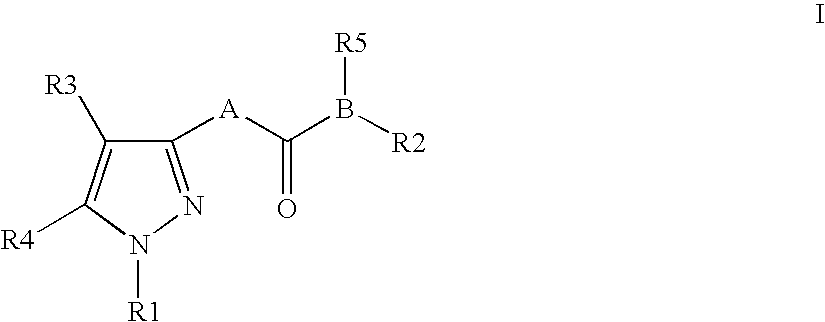
Pyrazole derivatives as cannabinoid receptor antagonists
InactiveUS20060100208A1Inhibit bindingBlock biological actionBiocideOrganic chemistryPyrimidine analogueChemistry
One aspect of the invention is concerned with cannabimimetic pyrazole analogs. Another aspect of the invention is concerned with new and improved pyrazole analogs having high affinities and / or selectivities for the CB1 cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
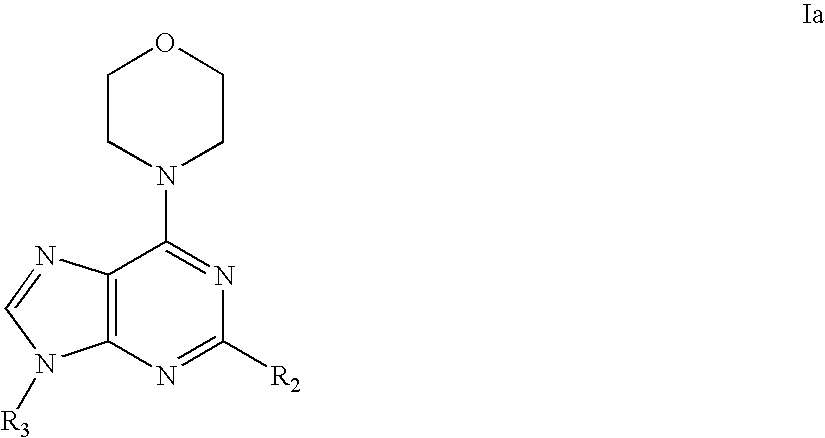
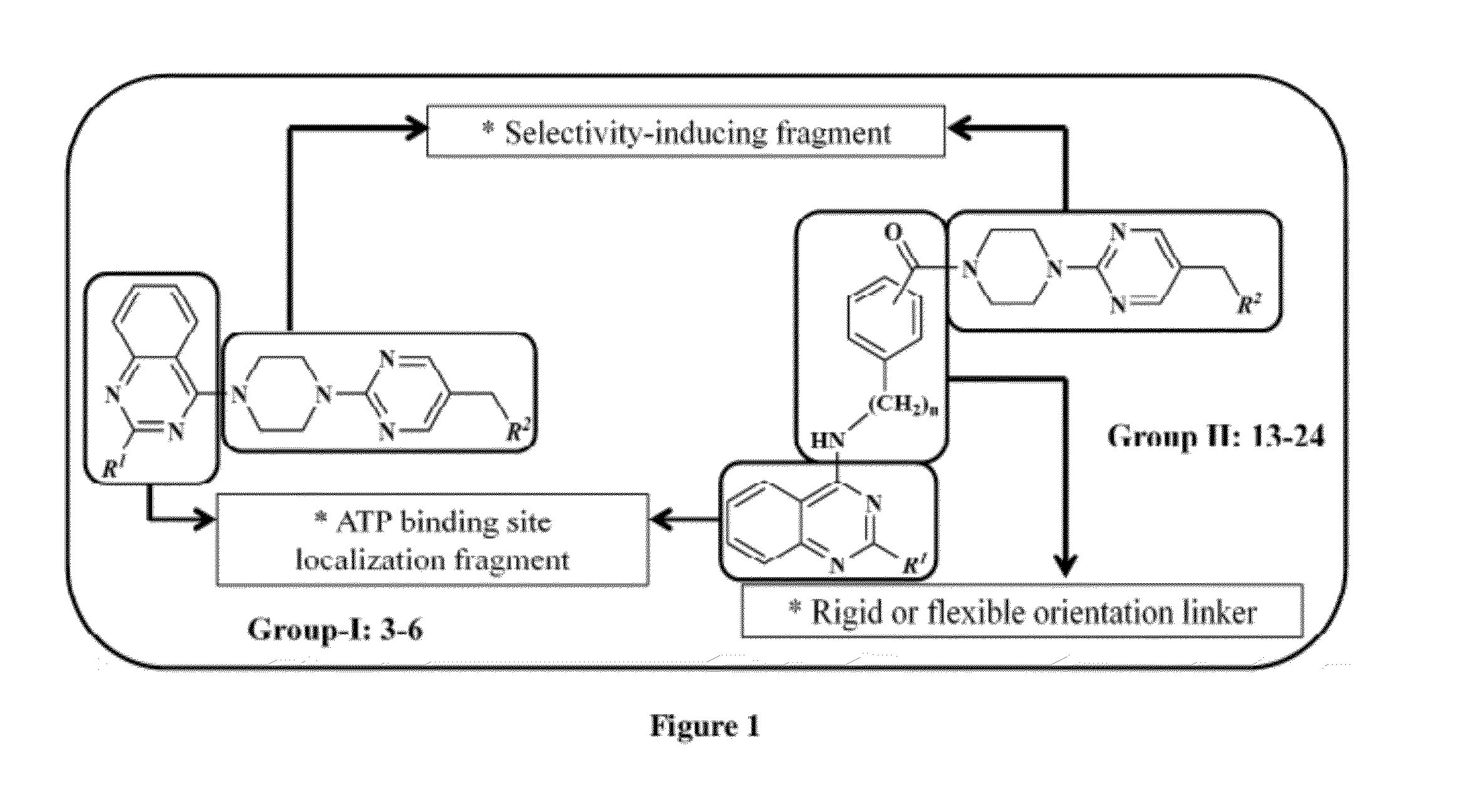
Imidazolopyrimidine analogs and their use as pi3 kinase and mtor inhibitors
The present invention relates to Imidazolopyrimidine Analogs, methods of making Imidazolopyrimidine Analogs, compositions comprising an Imidazolopyrimidine Analog, and methods for treating or preventing a PI3K-related disorder comprising administering to a subject in need thereof an effective amount of an Imidazolopyrimidine Analog. The invention also relates to methods for treating or preventing mTOR-related disorders comprising administering to a subject in need thereof an effective amount of an Imidazolopyrimidine Analog.
Owner:WYETH LLC
Pyrazole analogs acting on cannabinoid receptors
InactiveUS7393842B2Inhibit bindingBlock biological actionBiocideNervous disorderPyrimidine analogueCannabinoid receptor
One aspect of the invention is concerned with cannabimimetic pyrazole analogs. Another aspect of the invention is concerned with new and improved pyrazole analogs having high affinities and / or selectivities for the CB1 cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
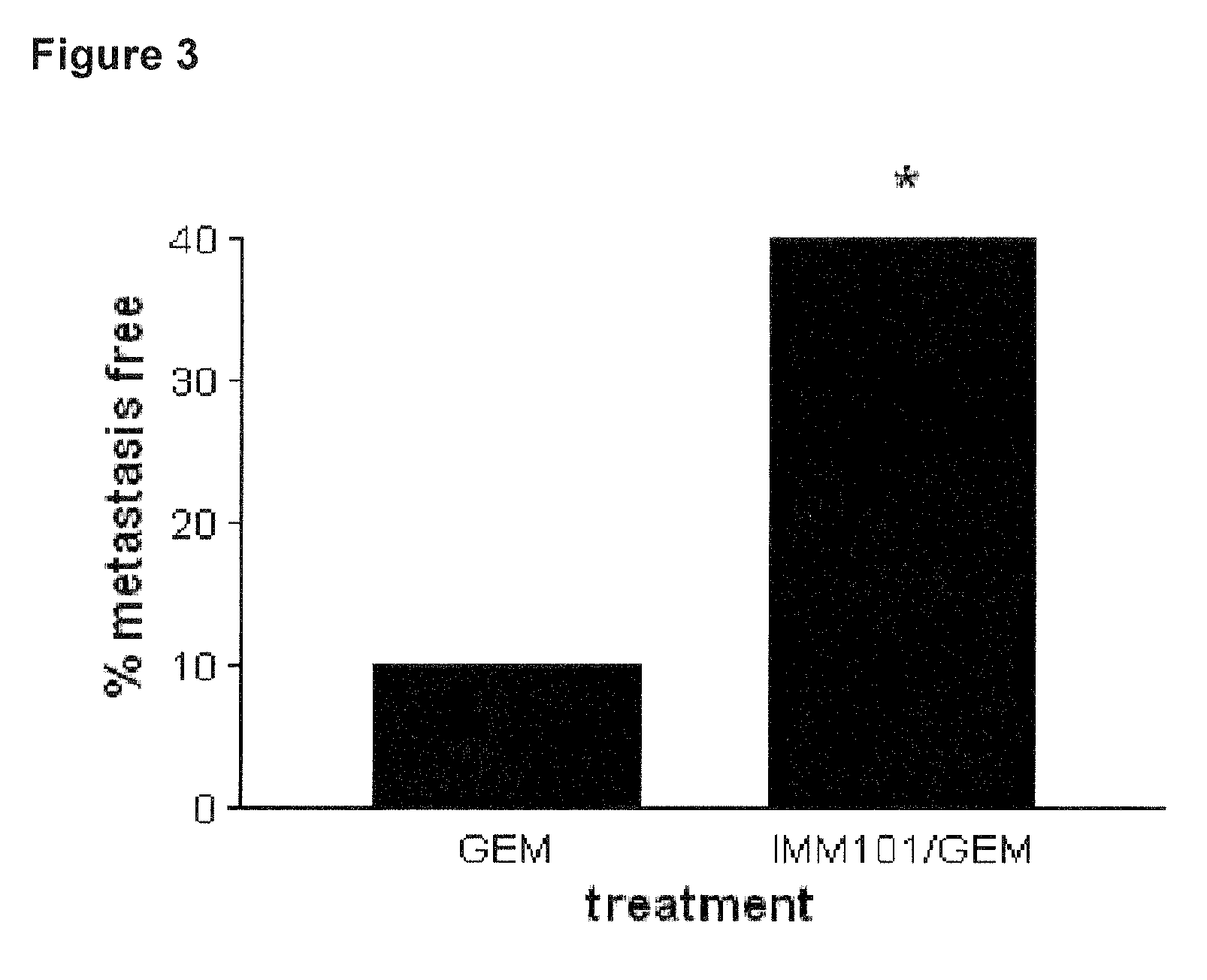
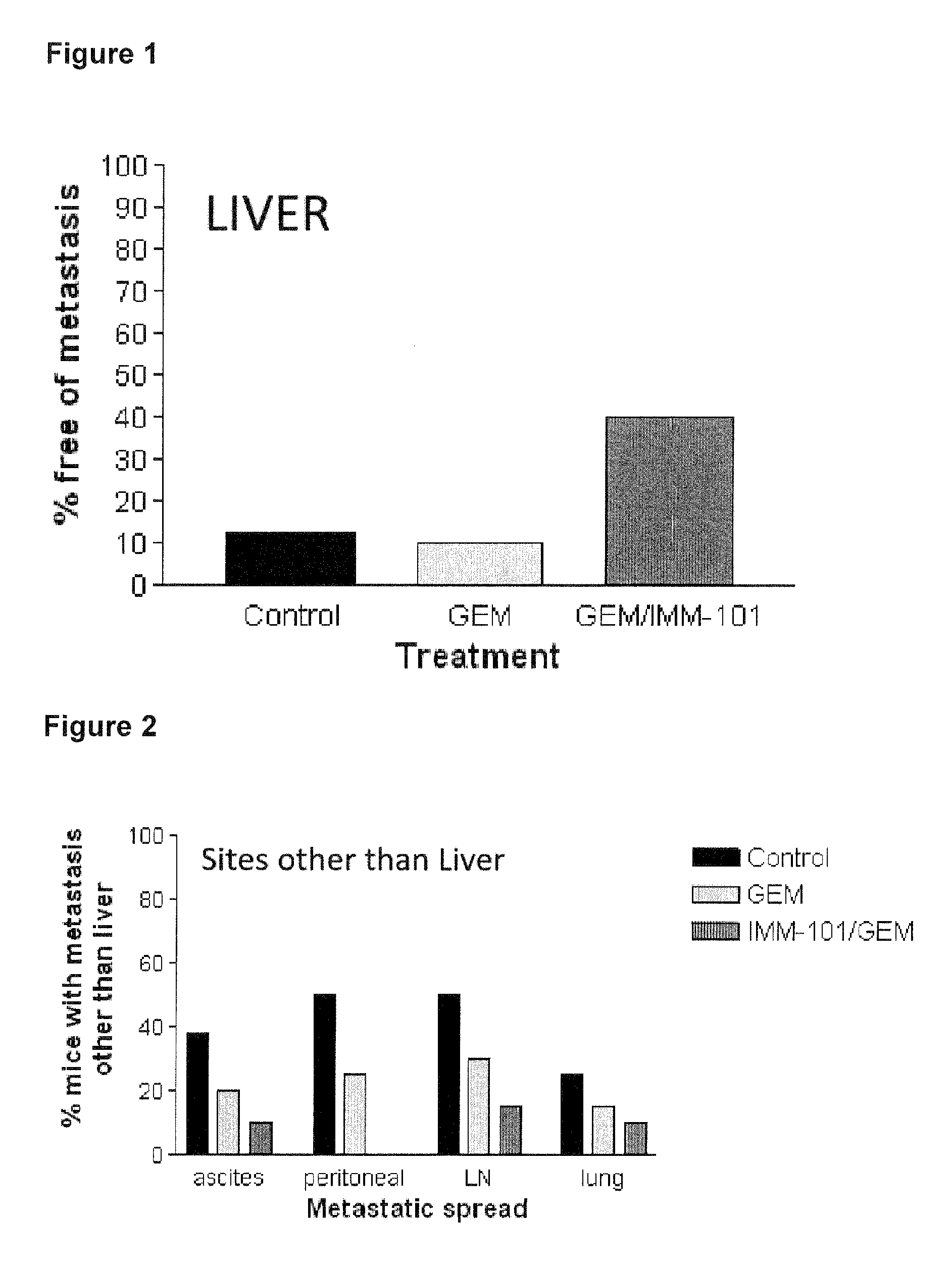
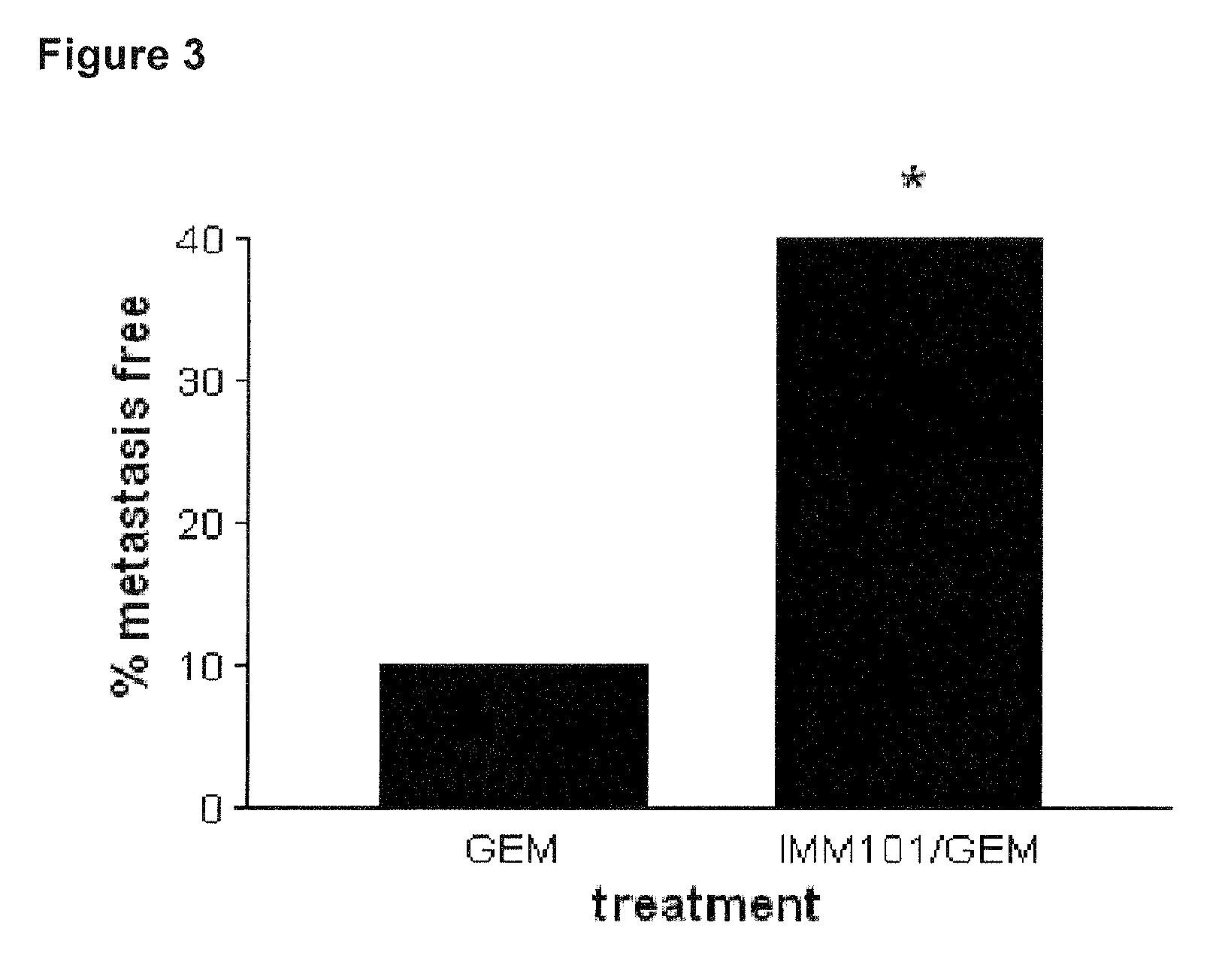
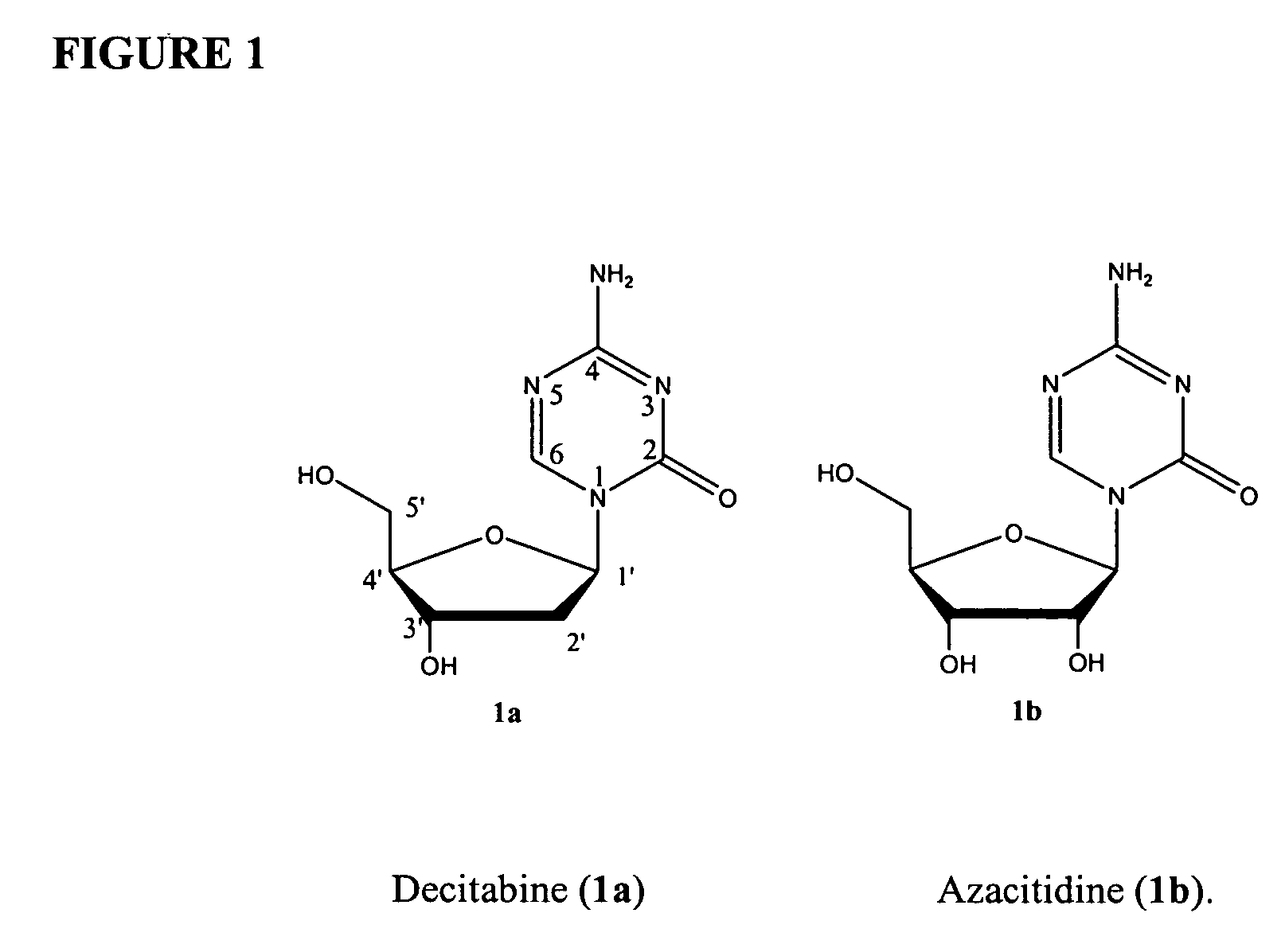
Cancer therapy
ActiveUS20130209517A1Great therapeutic responseBalanced immunoregulatoryBiocideBacterial antigen ingredientsParanasal Sinus CarcinomaPyrimidine analogue
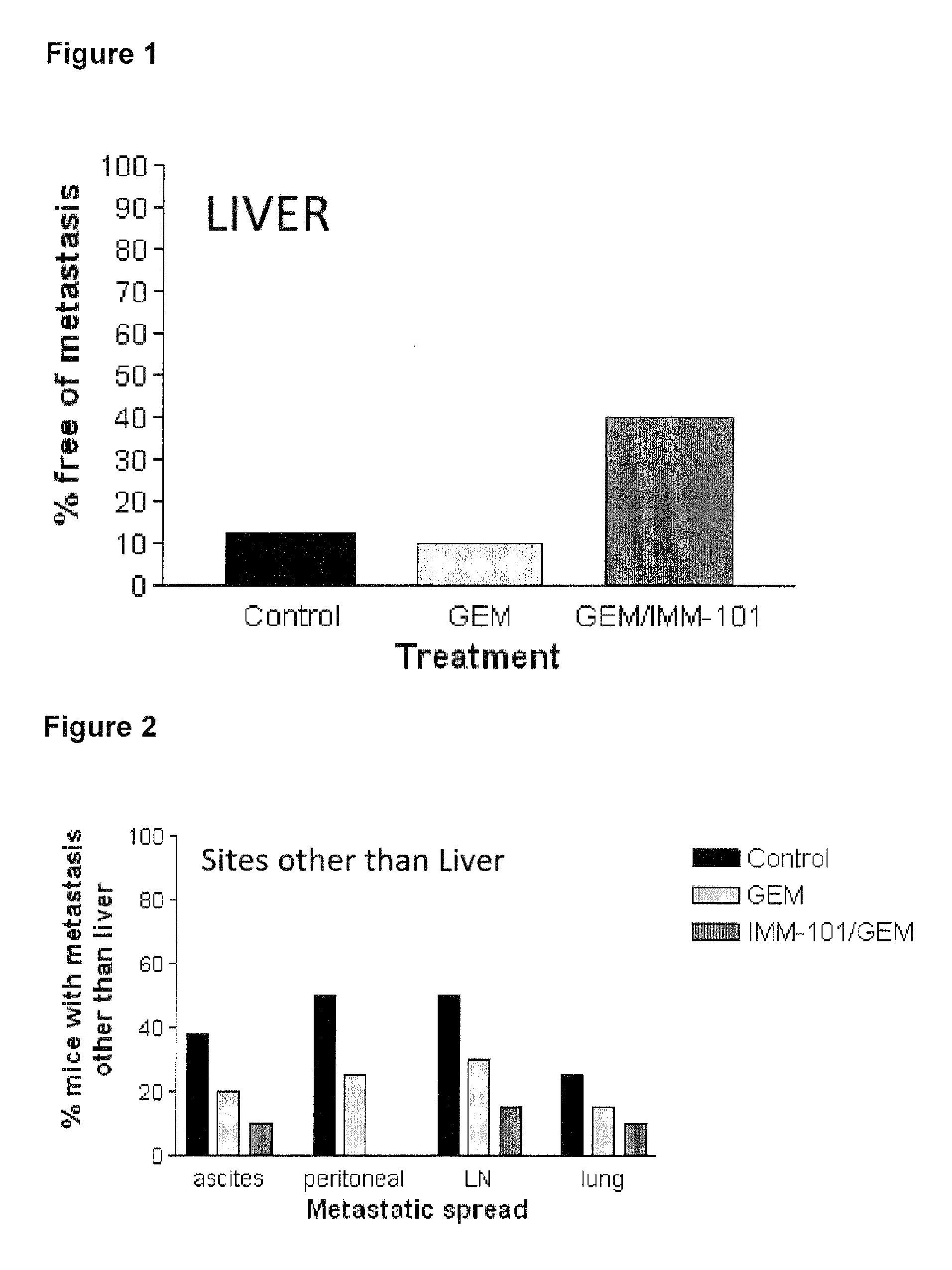
The present invention relates to a method of preventing, treating or inhibiting the development of tumors or metastases in a subject and to an immunomodulator for use in such therapy, in combination with a chemotherapeutic agent. An aspect the present invention is a method of preventing, treating, reducing, inhibiting and / or controlling the formation or establishment of metastasis of a primary neoplasia, tumor or cancer at one or more sites distinct from a primary neoplasia, tumor or cancer, in a subject intended to undergo chemotherapy, wherein the method comprises administering to the subject, a therapeutically effective amount of an antimetabolite pyrimidine analogue and an immunomodulator.
Owner:IMMODULON THERAPEUTICS
Azacytosine analogs and derivatives
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′–6′ position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
Azacytosine analogs and derivatives
InactiveUS20060205687A1Decrease electrophilicityBiocideSugar derivativesAbnormal tissue growthPyrimidine analogue
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 2-, 4-, or 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of using, synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease associated with aberrant DNA methylation, or a disease or condition that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders, tumors and cancers.
Owner:SUPERGEN
Novel pyrazole analogs acting on cannabinoid receptors
InactiveUS20060030563A1Inhibit bindingBlock biological actionBiocideOrganic chemistryPyrimidine analoguePyrazole
One aspect of the invention is concerned with cannabimimetic pyrazole analogs. Another aspect of the invention is concerned with new and improved pyrazole analogs having high affinities and / or selectivities for the CB1 cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
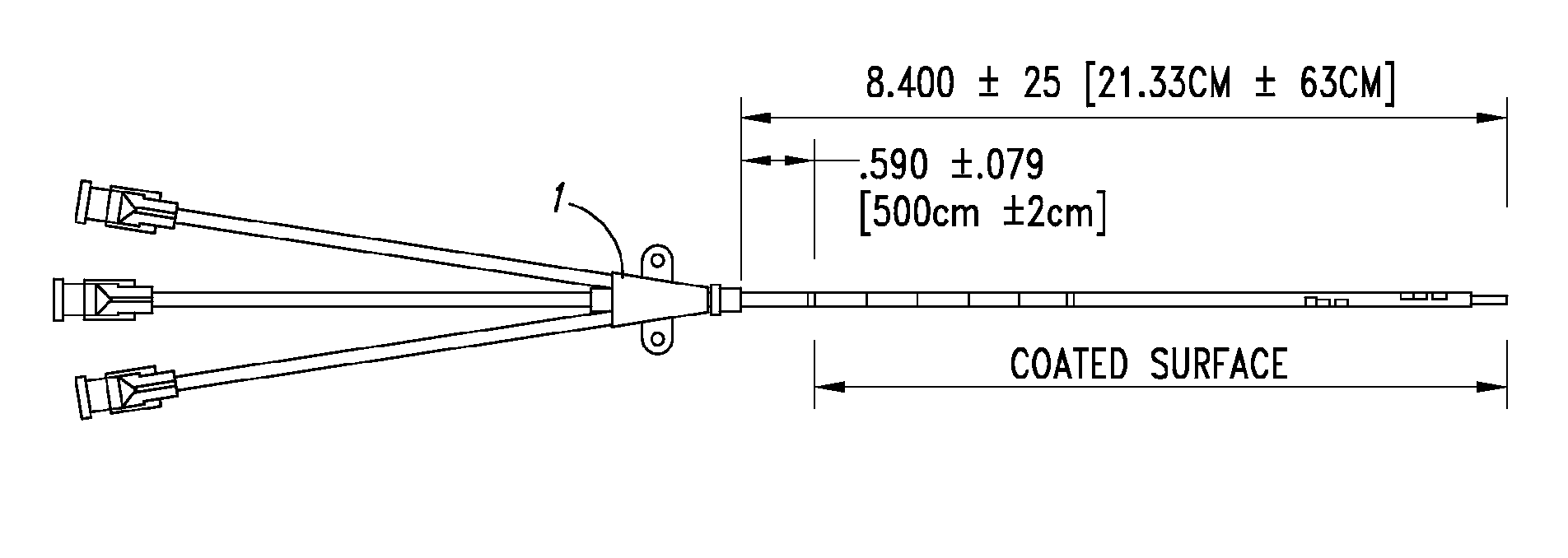
Anti-infective catheters
InactiveUS20110150961A1Reducing and inhibiting infectionBiocideTracheal tubesPyrimidine analogueCellulose
Anti-infective catheters are provided. Such catheters comprise a composition that comprises a pyrimidine analog, polyurethane, and cellulose or a cellulose-derived polymer, for example, in form of a coating. In addition, anti-infective compositions and methods of making and using anti-infective catheters are provided.
Owner:ANGIOTECH INT AG (CH)
Anticancer sustained release agent containing epothilone
InactiveCN1969816AEasy injectionIncrease drug concentrationOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolDepressant
Disclosed is an anti-cancer drugs slow release agent containing Epothilone which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include Epothilone, Epothilone derivatives, Epothilone B, Epothilone D and combination of anti-cancer drugs selected from phosphoinositide-3-kinase inhibitor, of pyrimidine analogues and / or DNA restoring enzyme inhibitor, the slow release auxiliary materials include polylactic acid and its copolymer, polyethylene glycol, PLA-COOH copolymer, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid), poly(fumaric acid-sebacylic acid), Polifeprosan, polylactic acid and other biocompatible high polymers, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose. The anticancer active constituents and the slow release microspheres can also be prepared into slow release implanting agent for intra-tumor or around-tumor injection or placement for the effective suppression of tumor growth and for the appreciable enhancement for curative effects of non-operative treatments such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
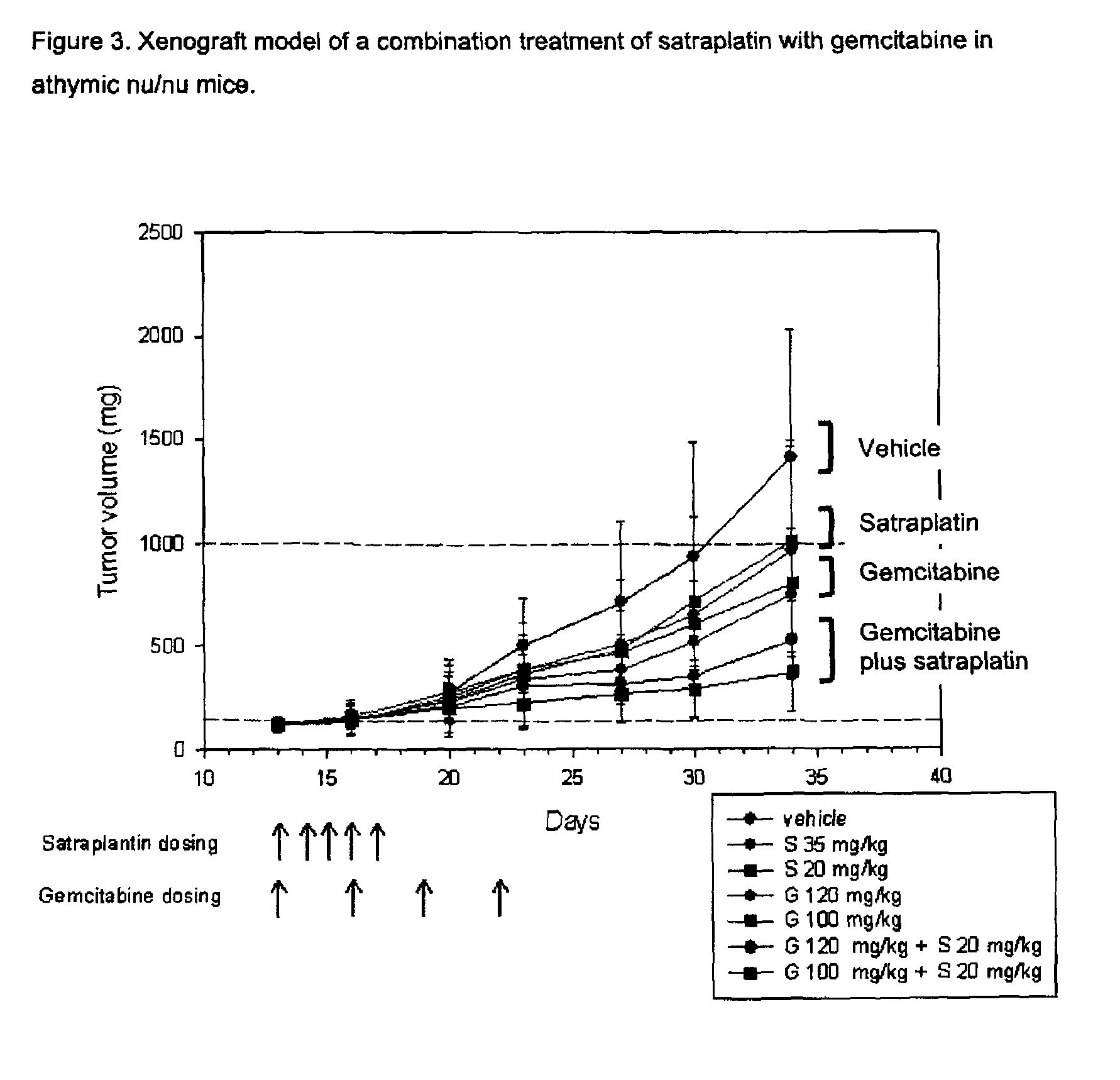
Anti-proliferative combination therapy using certain platinum-based chemotherapeutic agents and EGFR inhibitors or pyrimidine analogues
InactiveUS8048888B2Organic active ingredientsHeavy metal active ingredientsPlatinumPyrimidine analogue
The present invention describes a method or uses of prevention and / or treatment of a cancer or a tumor, and in particular to a combination therapy, methods, compositions and pharmaceutical packages comprising an inhibitor of receptors of the EGFR family or a chemotherapeutically active pyrimidine analogue and certain platinum-based chemotherapeutic agents.
Owner:AGENNIX
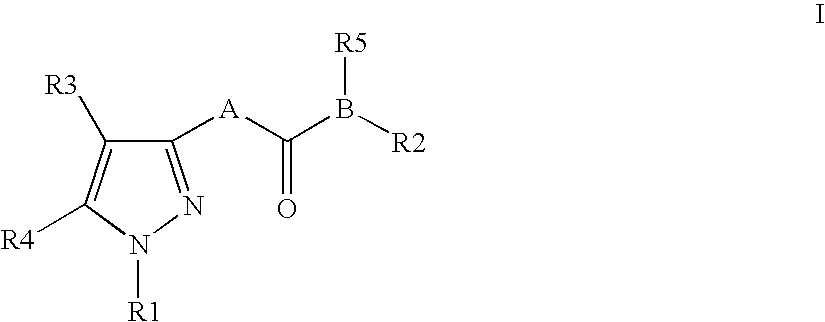
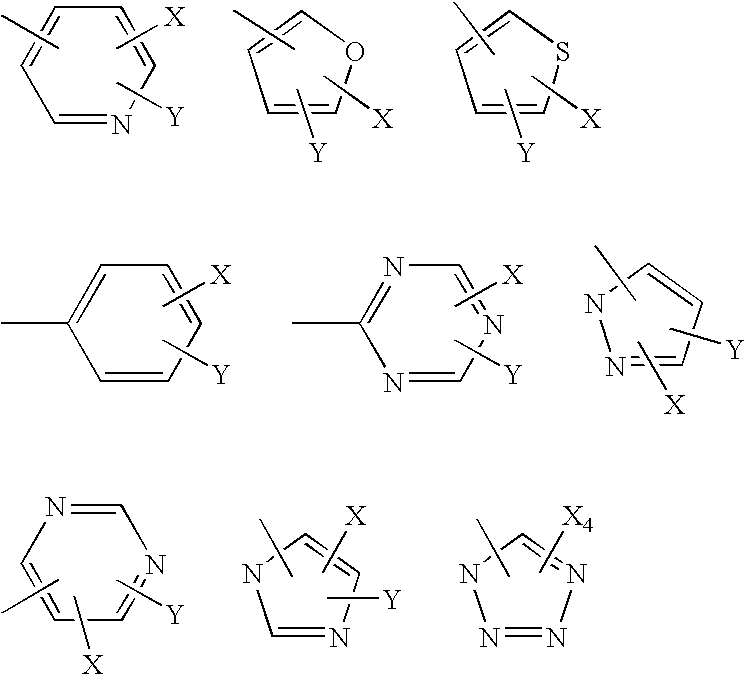
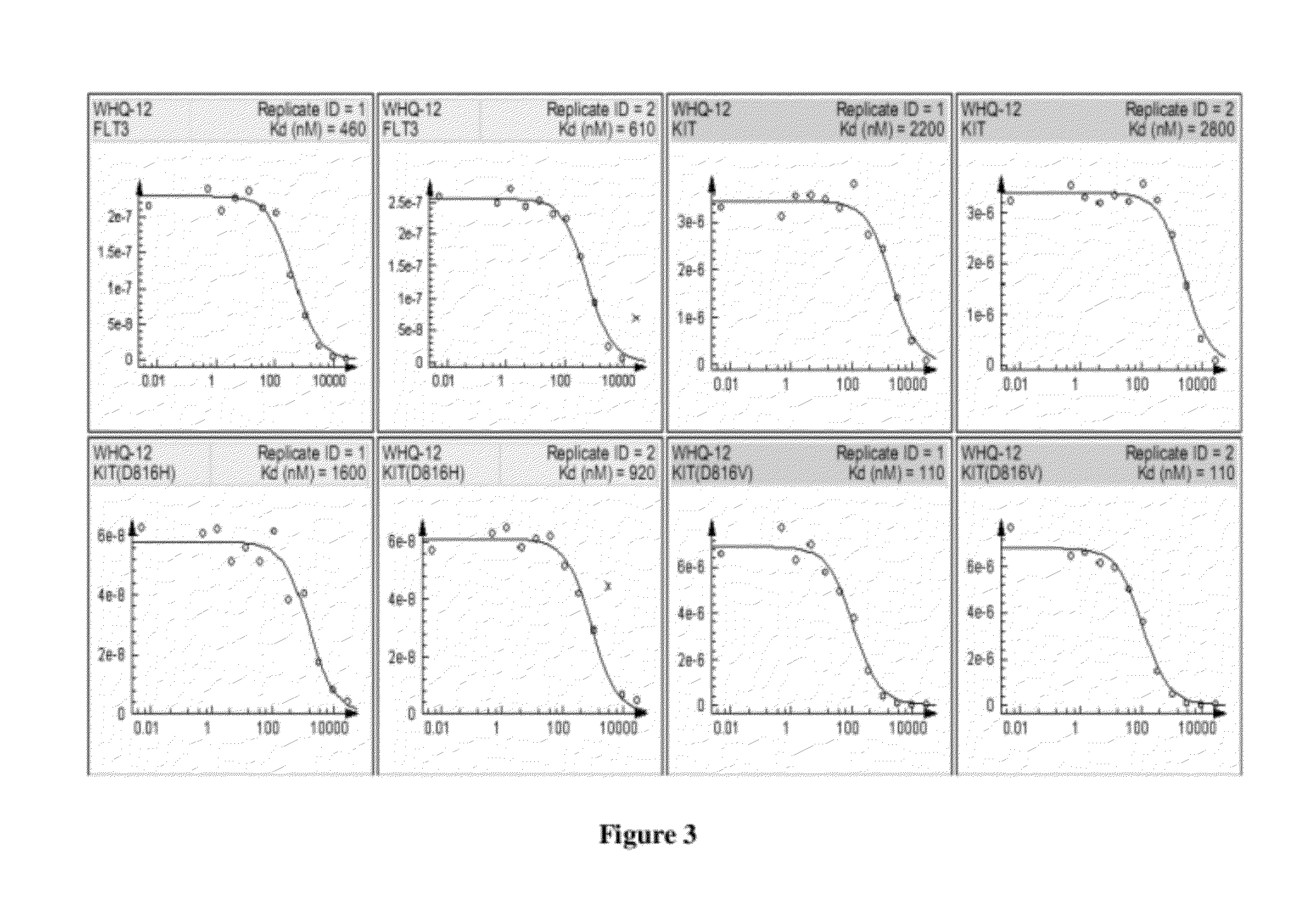
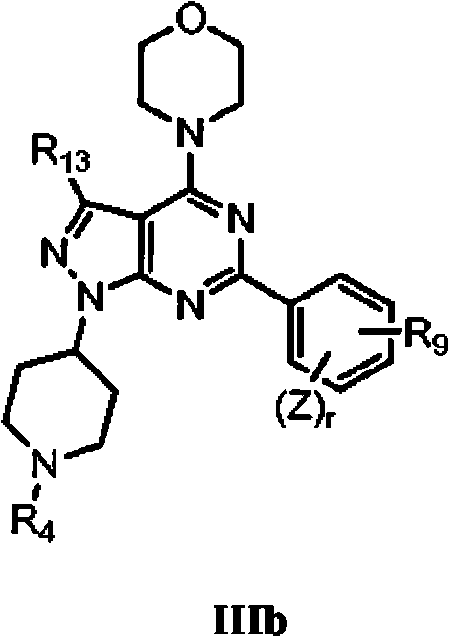
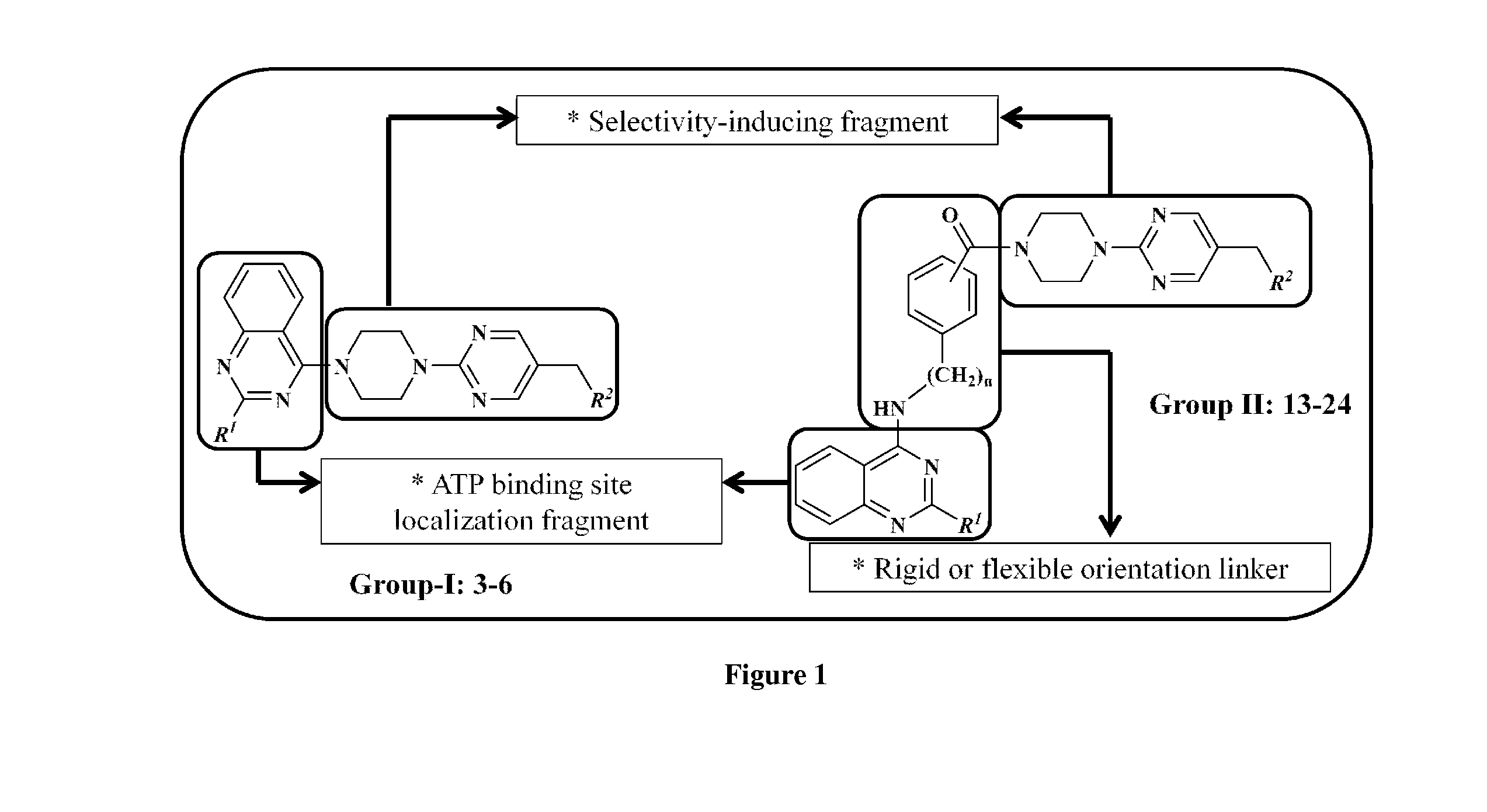
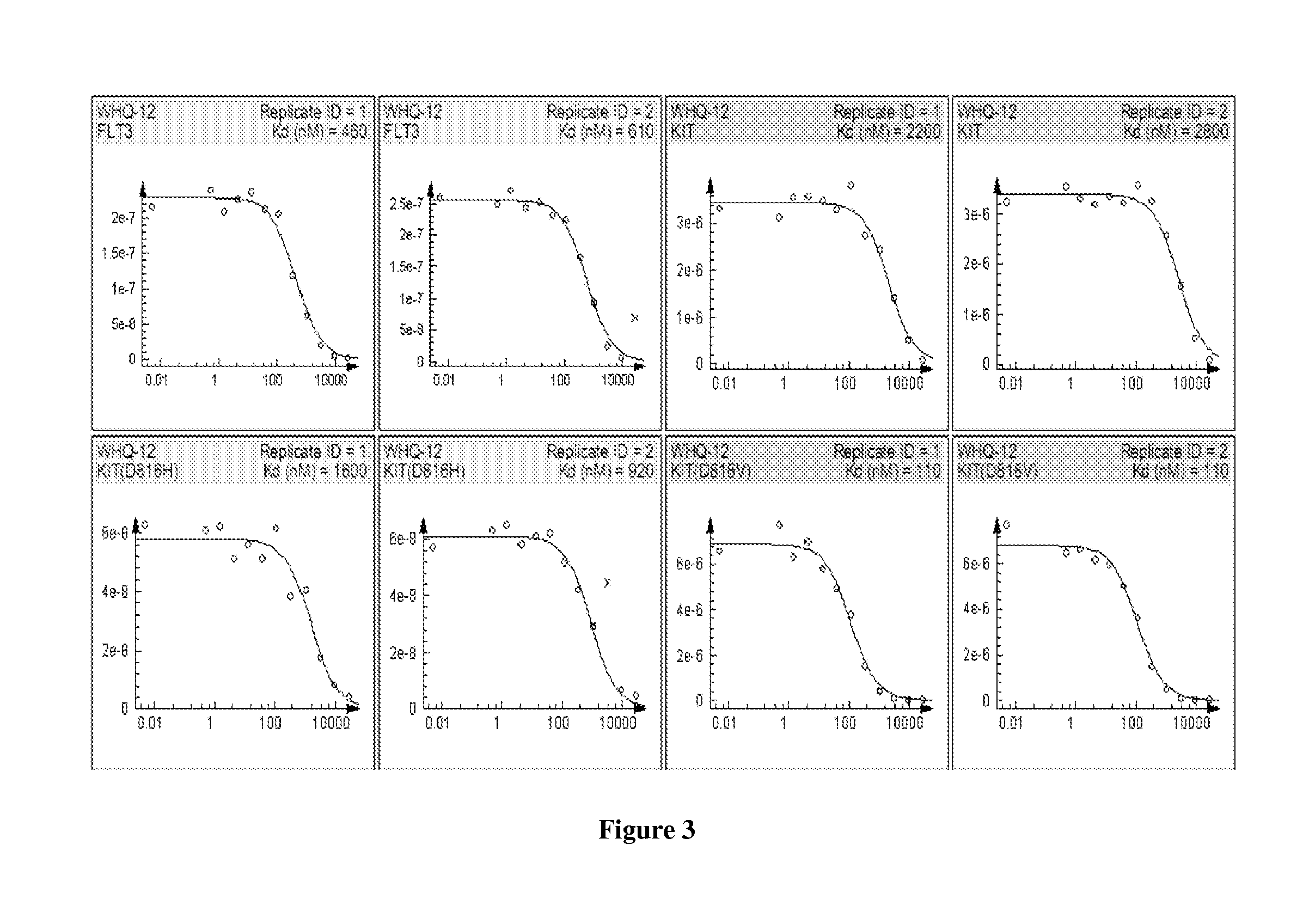
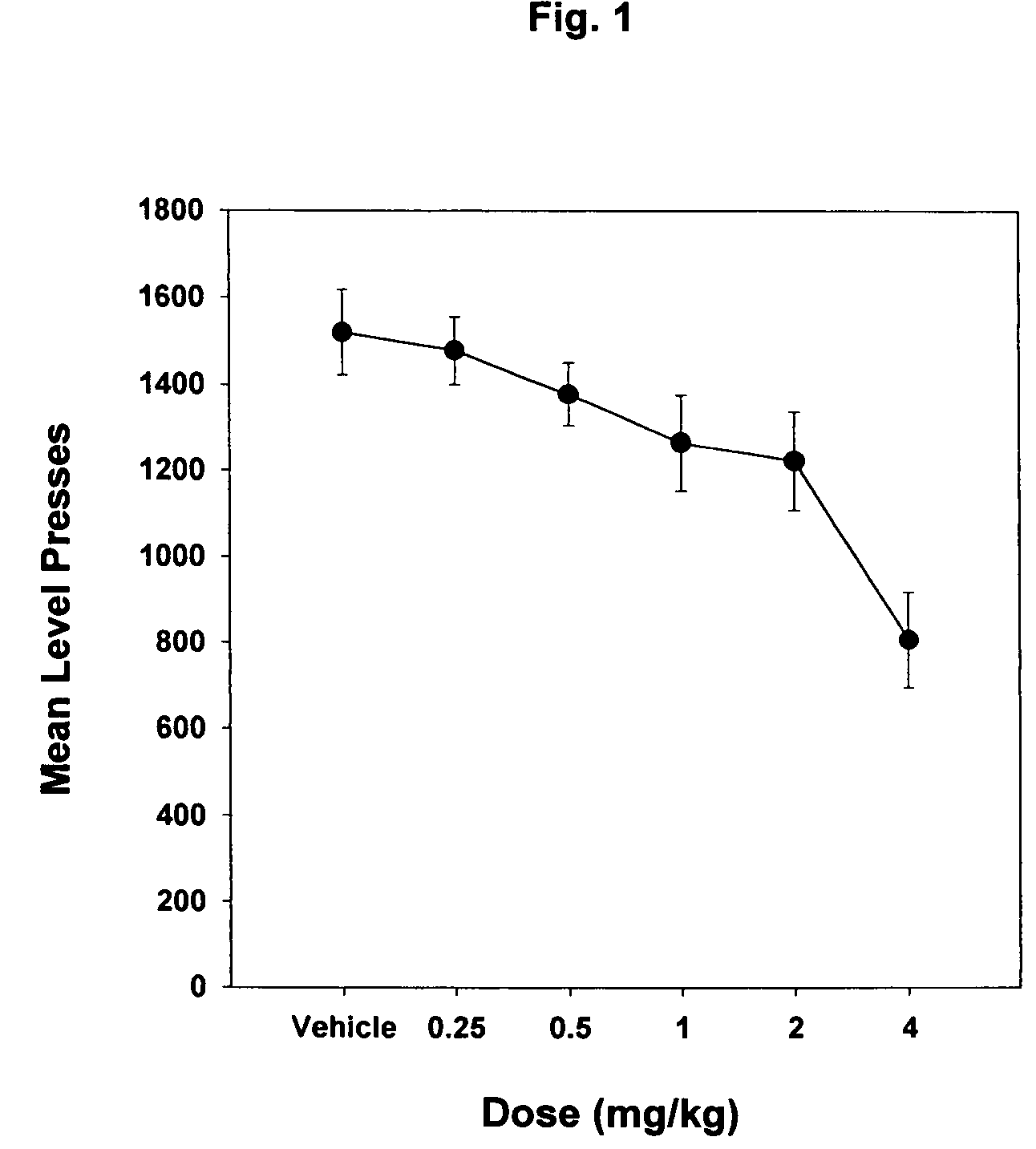
Piperazinylpyrimidine analogues as protein kinase inhibitors
The invention provides novel compounds based on piperazinylpyrimidine derivatives to be used as protein kinase inhibitors. The compounds may be useful in treating or preventing different cellular proliferation disorders, such as cancer. The present invention also provides methods of preparing these compounds, and methods of using the same.
Owner:UNIVERSITY OF THE PACIFIC
Pyrazolopyrimidine analogs and their use as mtor kinase and pi3 kinase inhibitors
The present invention relates to Pyrazolopyrimidine Analogs, methods of making Pyrazolopyrimidine Analogs, compositions comprising a Pyrazolopyrimidine Analog, and methods for treating mTOR -related disorders comprising administering to a subject in need thereof an effective amount of a Pyrazolopyrimidine Analog. The invention also relates to treating PI3K -related disorders comprising administering to a subject in need thereof an effective amount of a Pyrazolopyrimidine Analog.
Owner:WYETH LLC
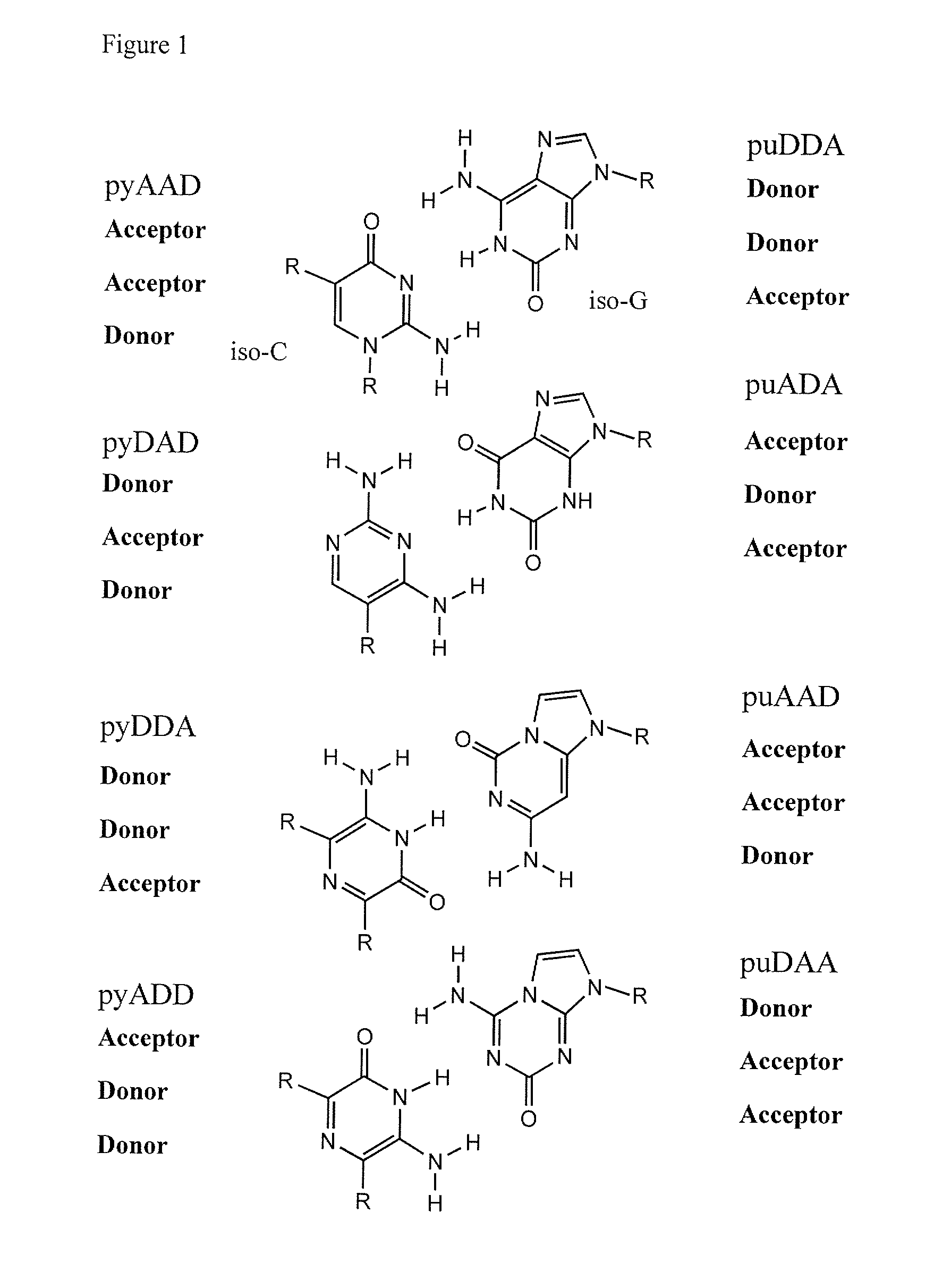
Ribonucleoside analogs with novel hydrogen bonding patterns
This invention relates to nucleoside, nucleotide, and oligonucleotide analogs that incorporate non-standard nucleobase analogs, defined to be those that present a pattern of hydrogen bonds to a paired nucleobase analog in a complementary strand that is different from the pattern presented by adenine, guanine, cytosine, and thymine. The invention is specifically concerned with nucleotide analogs that present the donor-donor-acceptor, hydrogen bonding patterns on pyrimidine analogs, and especially those that are analogs of ribonucleotides, including protected ribonucleotides suitable for phosphoramidite-based synthesis of RNA. The heterocycles on these nucleoside analogs are aminopyridones that have electron withdrawing groups attached to the position analogous to the 5-position of the ring in standard pyrimidines, including nitro, cyano, and carboxylic acid derivatives.
Owner:BENNER STEVEN A +1
Slow-released injection containing methotrexate and its synergist
The slow released anticancer injection containing methotrexate and its synergist consists of slow released microballoon and solvent. The slow released microballoon includes effective anticancer component and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The effective anticancer component is methotrexate synergist or the composition of methotrexate and its synergist; the methotrexate synergist is selected from phosphorinositide 3-kinase (PI3K) inhibitor, pyrimidine analogue and DNA repair enzyme inhibitor; the slow releasing supplementary material is PLA, PLGA, EVAc, etc or their composition; and the suspending agent is sodium carboxymethyl cellulose, etc. The slow released microballoon may be also prepared into slow released implantation preparation. Implanting or injecting the slow released preparation to local tumor part can lower the systematic toxic reaction of the medicine and raise the medicine concentration of local tumor part selectively to raise the treating effect.
Owner:JINAN SHUAIHUA PHARMA TECH
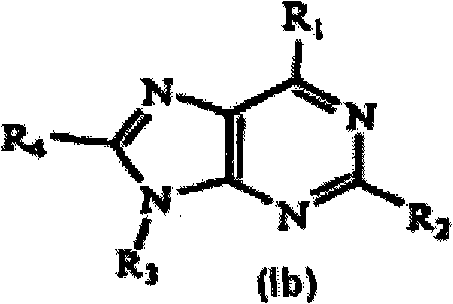
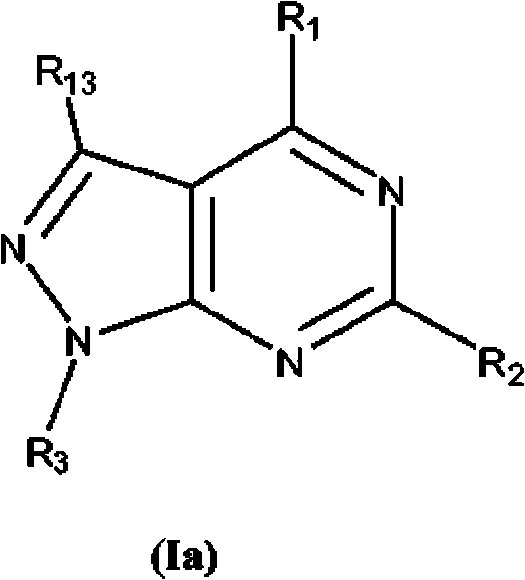
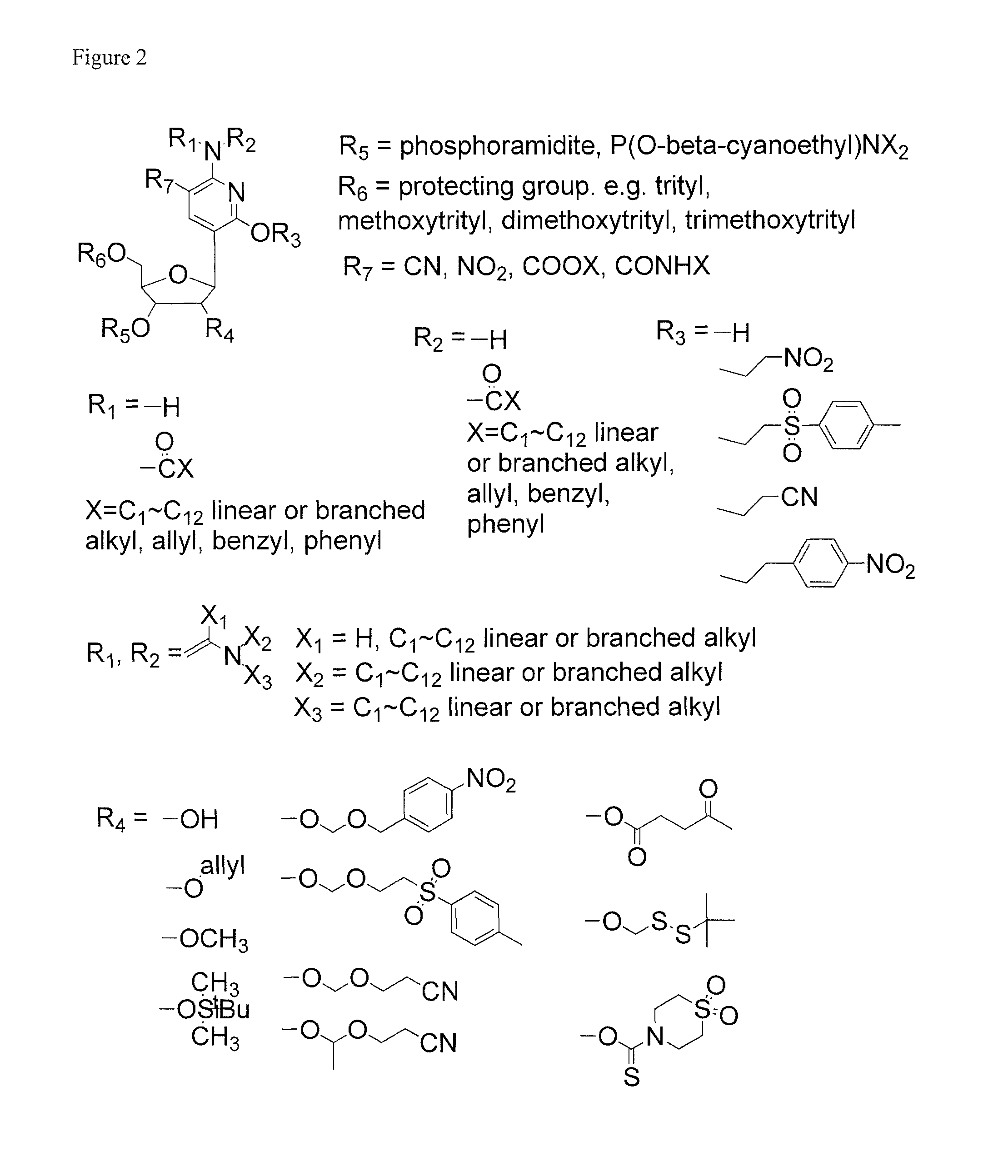
Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses
The present invention is in the field of nucleic acid binding compounds comprising 7-substituted 7-deaza-8aza-2,6-diamino-purine bases, compounds useful for the preparation of such compounds, various uses thereof and methods for the determination of nucleic acids using said compounds in the field of diagnostics.
Owner:ROCHE MOLECULAR SYST INC
Pyrazole derivatives as cannabinoid receptor antagonists
One aspect of the invention is concerned with cannabimimetic pyrazole analogs. Another aspect of the invention is concerned with new and improved pyrazole analogs having high affinities and / or selectivities for the CB1 cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
Anti-infective catheters
Owner:ANGIOTECH INT AG (CH)
Piperazinylpyrimidine analogues as protein kinase inhibitors
The invention provides novel compounds based on piperazinylpyrimidine derivatives to be used as protein kinase inhibitors. The compounds may be useful in treating or preventing different cellular proliferation disorders, such as cancer. The present invention also provides methods of preparing these compounds, and methods of using the same.
Owner:UNIVERSITY OF THE PACIFIC
Pyrazole analogs acting on cannabinoid receptors
InactiveUS7745440B2Less lipophilicShorter in vivo half-livesBiocideOrganic chemistryPyrimidine analogueCannabinoid receptor
One aspect of the invention is concerned with cannabimimetic pyrazole analogs. Another aspect of the invention is concerned with new and improved pyrazole analogs having high affinities and / or selectivities for the CB1 cannabinoid receptor. A further aspect of the invention is concerned with pharmaceutical preparations employing the inventive analogs and methods of administering therapeutically effective amounts of the inventive analogs to provide a physiological effect.
Owner:UNIV OF CONNECTICUT
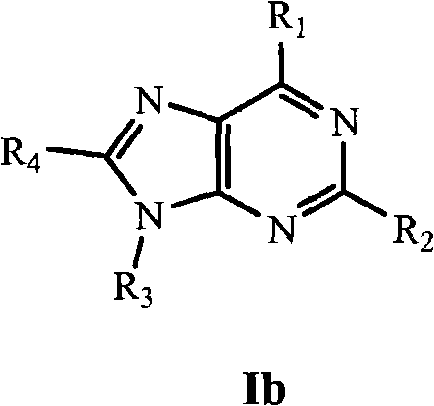
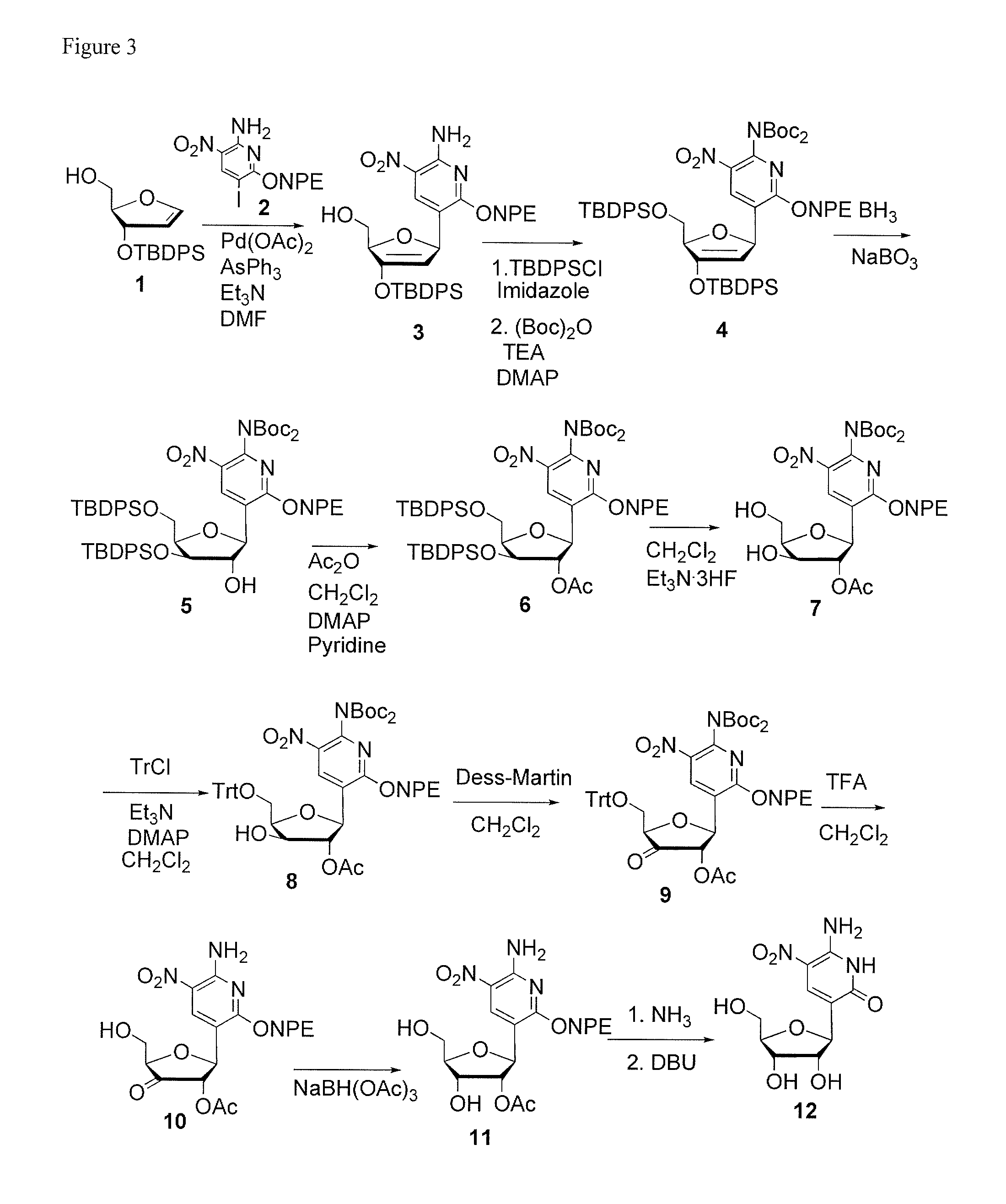
Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses
ActiveUS20110021365A1Improve solubilityOrganic active ingredientsSugar derivativesPyrimidine analoguePurine
The present invention is in the field of nucleic acid binding compounds comprising 7-substituted 7-deaza-8aza-2,6-diamino-purine bases, compounds useful for the preparation of such compounds, various uses thereof and methods for the determination of nucleic acids using said compounds in the field of diagnostics.
Owner:ROCHE MOLECULAR SYST INC
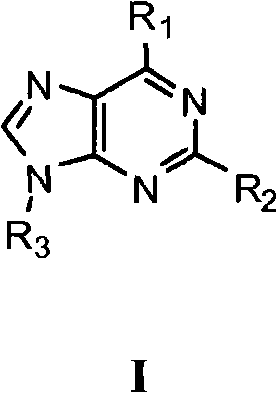
Modulators of cell cycle checkpoints and their use in combination with checkpoint kinase inhibitors
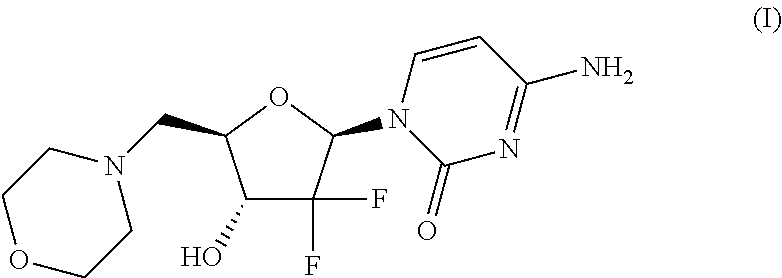
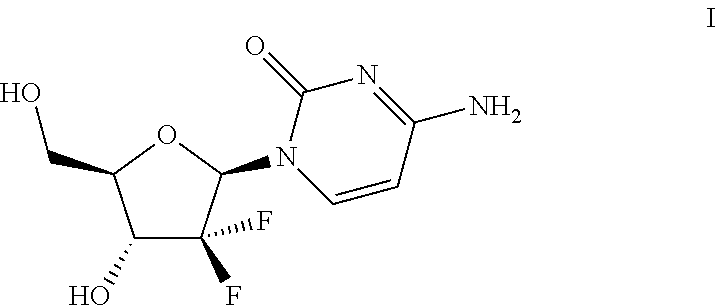
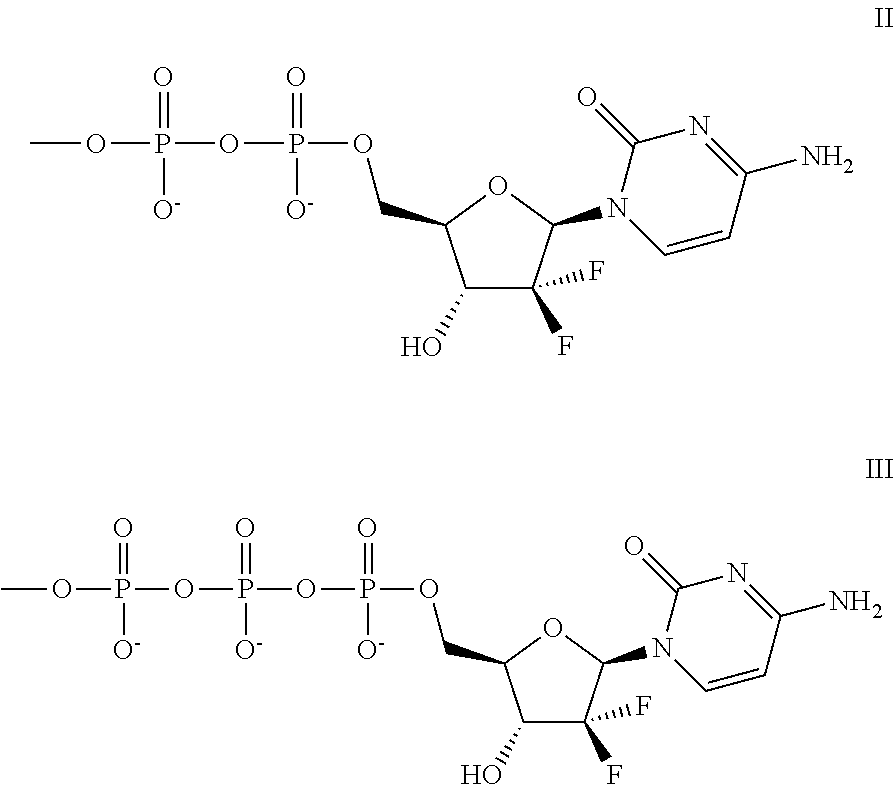
In its many embodiments, the present invention provides a novel class of pyrimidine analogs as targeted mechanism-based modulators of cell cycle checkpoints. Cancers and / or malignancies can be treated by administration of a cell cycle checkpoint modulator of the invention. Also discussed are suitable combinations of the cell cycle checkpoint modulator with a checkpoint kinase inhibitor to produce synergistic apoptosis in cancer cells. The invention also includes methods of treating cancers by administering the combination of the cell cycle checkpoint modulator and the checkpoint kinase inhibitor, pharmaceutical compositions comprising the cell cycle checkpoint modulator as well as combinations and pharmaceutical kits. An example cell cycle checkpoint modulator is shown below: formula (I).
Owner:MERCK SHARP & DOHME LLC
Cancer therapy
ActiveUS8617520B2Safe, better tolerated and effective methodImprove responseBiocideBacterial antigen ingredientsParanasal Sinus CarcinomaPyrimidine analogue
The present invention relates to a method of preventing, treating or inhibiting the development of tumors or metastases in a subject and to an immunomodulator for use in such therapy, in combination with a chemotherapeutic agent. An aspect the present invention is a method of preventing, treating, reducing, inhibiting and / or controlling the formation or establishment of metastasis of a primary neoplasia, tumor or cancer at one or more sites distinct from a primary neoplasia, tumor or cancer, in a subject intended to undergo chemotherapy, wherein the method comprises administering to the subject, a therapeutically effective amount of an antimetabolite pyrimidine analogue and an immunomodulator.
Owner:IMMODULON THERAPEUTICS
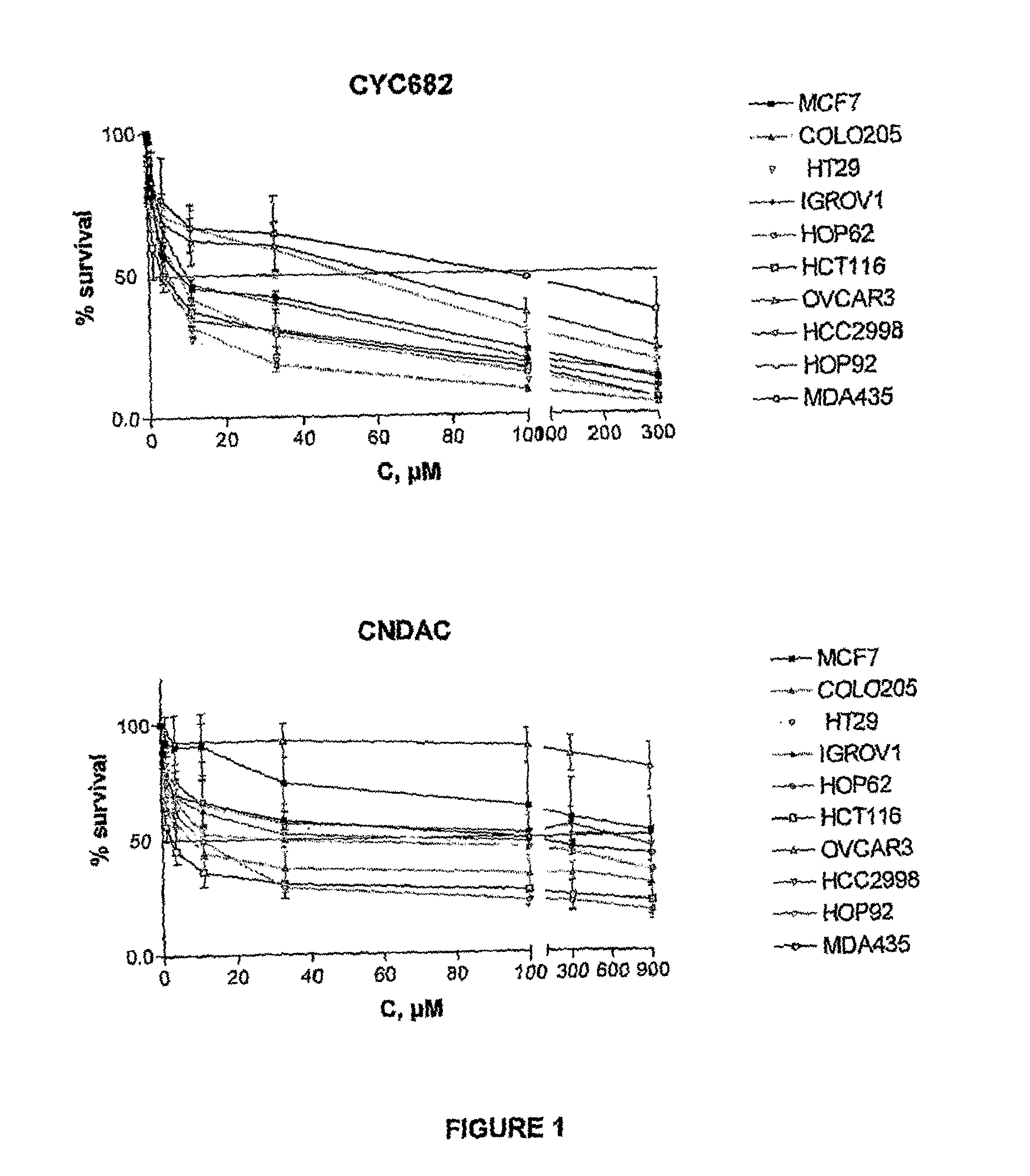
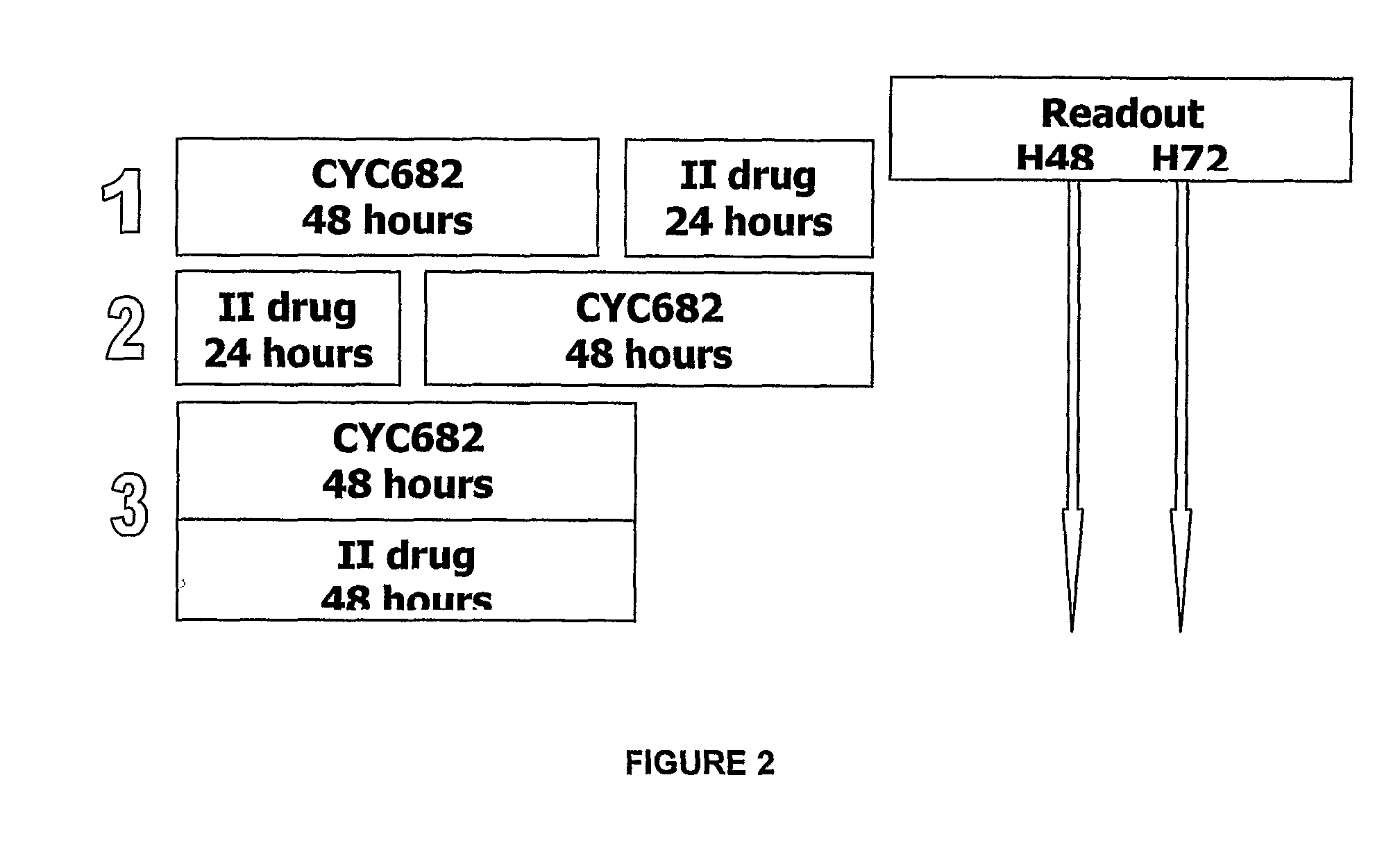
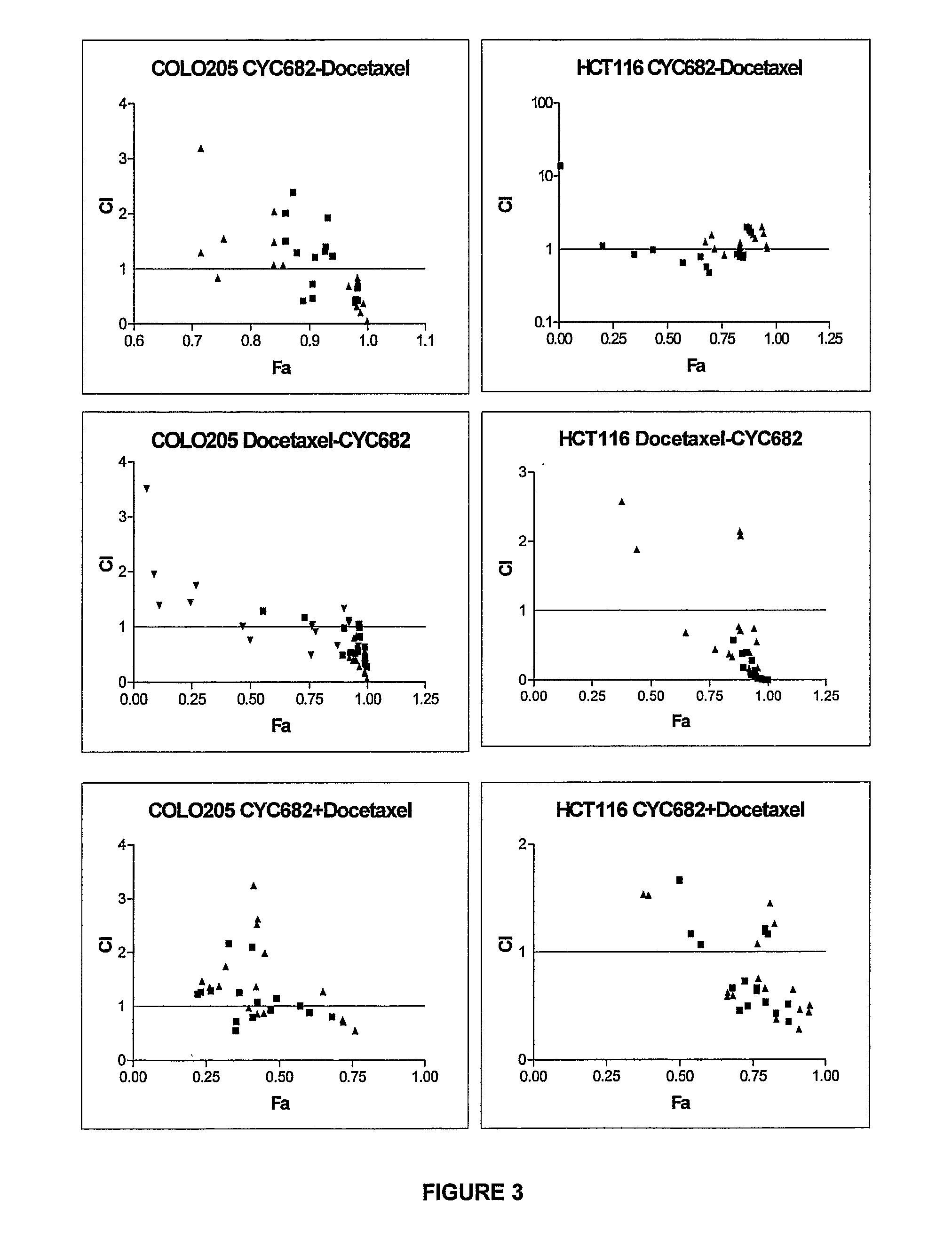
Antiproliferative combination comprising cyc-682 and a cytotoxic agent
A first aspect of the invention relates to a combination comprising 2′-cyano-2′-deoxy-N4-palmitoyl-1-beta-D-arabi-nofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from (a) a vinca alkaloid; (b) a taxane; (c) a cytosine analogue; (d) an anthracycline; and (e) a platinum antineoplastic agent. A second aspect of the invention relates to a pharmaceutical product comprising the above combination as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method for treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering the above combination.
Owner:CYCLACEL
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is a compound anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer effective ingredients include Aclarubicin, Idarubicin, Doxorubicin, Epirubicin, Valtaxin, Pirarubicin, Losaxantrone, Losoxantrone and / or anticancer antibiotic synergistic agents selected from phosphoinositide-3-kinase inhibitor, pyrimidine analogues and / or DNA restoration enzyme inhibitor, the slow release auxiliary materials are selected from polylactic acid copolymer EVAc, or sebacic acid copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C). The slow release microspheres can also be prepared into slow release implanting agent for lowering down the whole body toxicity reaction of the medicament when locally dispensing on the tumor, and for selectively increasing the tumor local medicinal concentration.
Owner:SHANDONG LANJIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

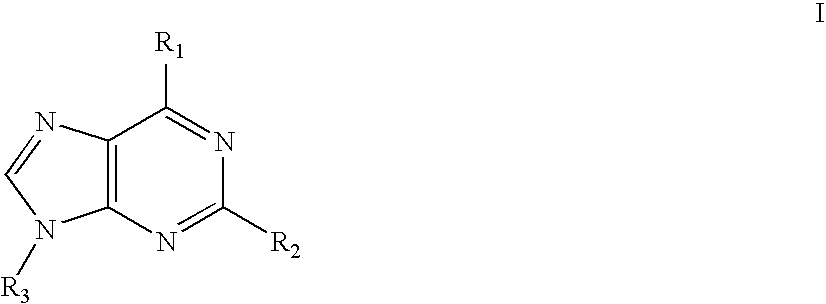
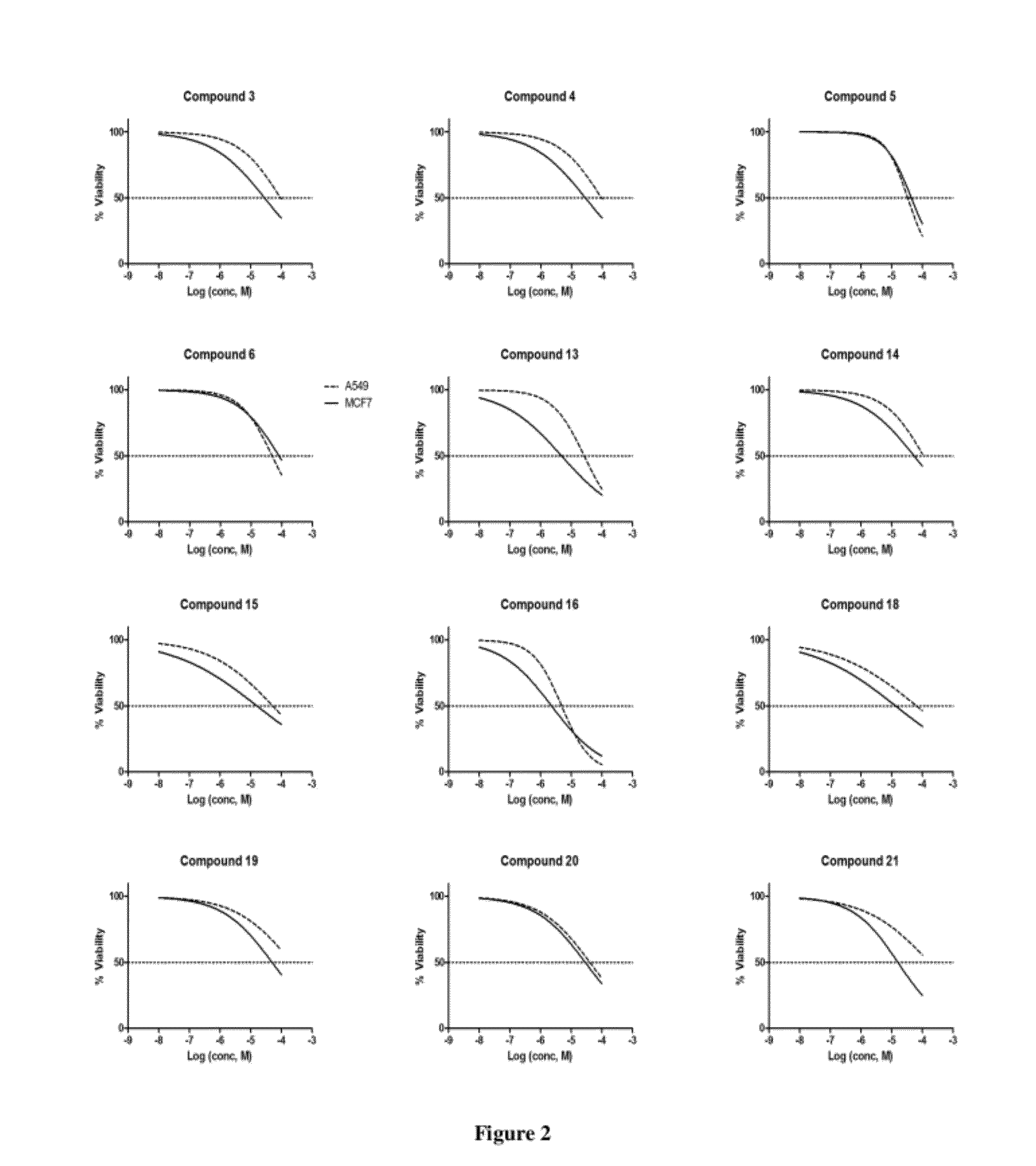
![Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses](https://images-eureka.patsnap.com/patent_img/a2927a0c-1128-4d3b-9a28-58dd4a4b9116/US07238795-20070703-D00000.png)
![Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses](https://images-eureka.patsnap.com/patent_img/a2927a0c-1128-4d3b-9a28-58dd4a4b9116/US07238795-20070703-D00001.png)
![Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses Nucleic acid binding compounds containing pyrazolo[3,4-d]pyrimidine analogues of purin-2,6-diamine and their uses](https://images-eureka.patsnap.com/patent_img/a2927a0c-1128-4d3b-9a28-58dd4a4b9116/US07238795-20070703-D00002.png)
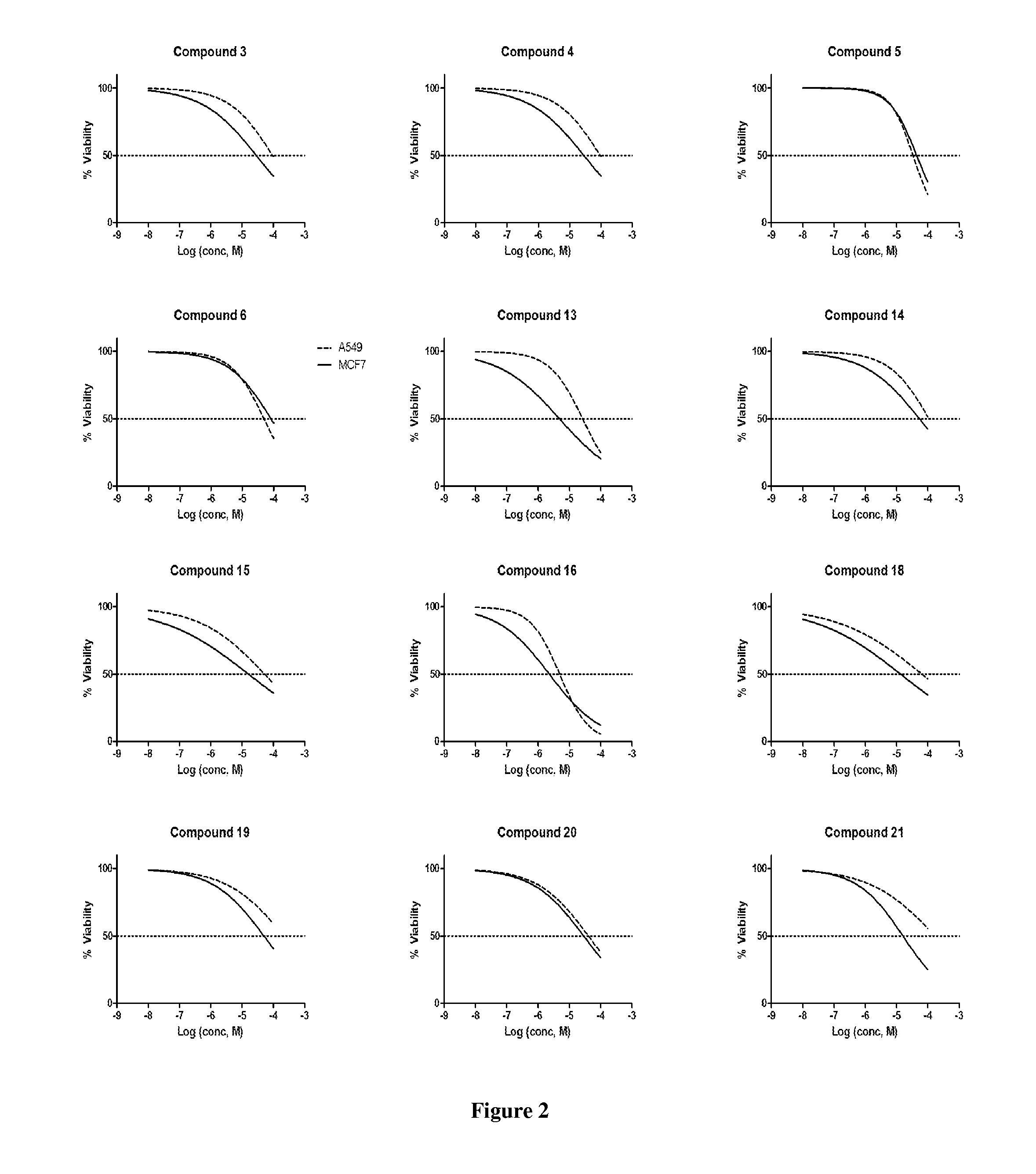
![Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses](https://images-eureka.patsnap.com/patent_img/e3b96518-6f45-4459-92f2-1b6caf1ba5a8/US20110021365A1-20110127-D00001.png)
![Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses](https://images-eureka.patsnap.com/patent_img/e3b96518-6f45-4459-92f2-1b6caf1ba5a8/US20110021365A1-20110127-D00002.png)
![Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses Nucleic Acid Binding Compounds Containing Pyrazolo[3,4-D]Pyrimidine Analogues of Purin-2,6-Diamine and Their Uses](https://images-eureka.patsnap.com/patent_img/e3b96518-6f45-4459-92f2-1b6caf1ba5a8/US20110021365A1-20110127-D00003.png)