Imidazolopyrimidine analogs and their use as pi3 kinase and mtor inhibitors
A compound, C1-C3 technology, used in anti-inflammatory agents, drug combinations, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[1085] general method
[1086] The following general procedures will outline the synthesis of imidazopyrimidine analogs for each example.
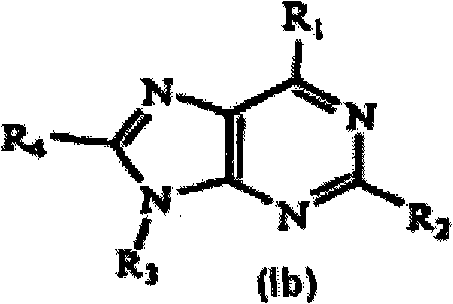
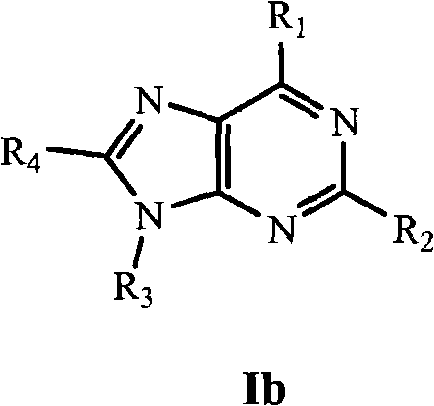
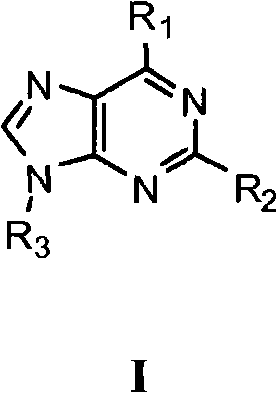
[1087] Synthesis of 6-morpholin-4-yl-2-aryl-9H-purine (Scheme 1)
[1088] Step 1: To a solution of 2,6-dichloropurine (0.8 g, 4.23 mmol) dissolved in EtOH (40 mL) was added morpholine (1.5 eq). The reaction was stirred at room temperature for 12 hours, and the crude solid product was filtered off. with Et 2 The crude material was washed with O and dried in vacuo to give 0.75 g of a beige solid.
[1089] To the morpholine product from Step 1 (50 mg, 0.21 mmol) dissolved in DMF (0.5 mL) was added the desired arylboronic acid (1.5 eq), Na 2 CO 3 solution (2eq) and Pd(PPh 3 ) 4 (catalytic amount). The reaction was heated at 175 °C for 10 min under microwave irradiation. The crude reaction was then concentrated and purified via preparative HPLC using a Gilson instrument (see below).
[1090] Synthesis of 6-morpholin-4-yl-2-aryl-9-pipe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com