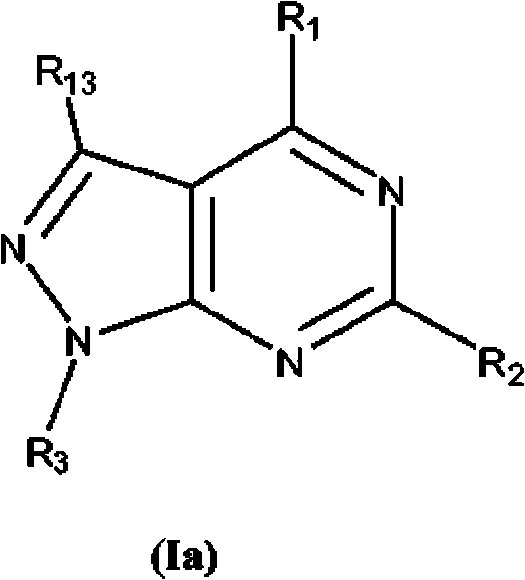
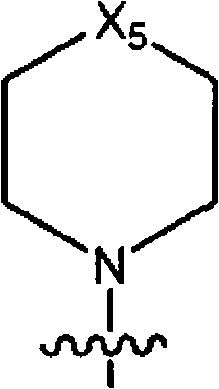
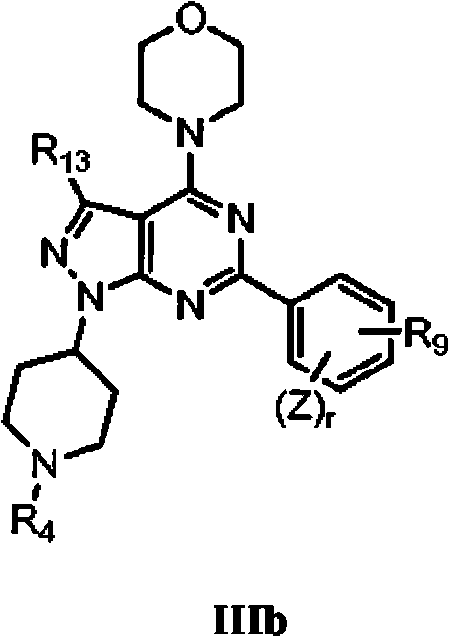
Pyrazolopyrimidine analogs and their use as mtor kinase and pi3 kinase inhibitors
A compound, C1-C3 technology, used in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as unclear activation mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[1366] general method
[1367] The following general procedures will outline the synthesis of the pyrazolopyrimidine analogs for each example.
[1368] Summary: Preparative HPLC using a Gilson instrument
[1369] The crude material was dissolved in 1.5 ml DMSO and 0.5 ml MeOH, filtered through 0.45 μm GMF, and purified by Gilson HPLC using Phenomenex LUNA C 18 Column: 60mm×21.20mm I.D., 5μm particle size, using ACN / water (containing 0.2% TFA or ammonium hydroxide) gradient elution. Appropriate fractions were subsequently analyzed by LC / MS.
[1370] Analytical HPLC conditions: instrument - Agilent 1100; column: Thermo Aquasil C18, 50×2.1mm, 5μm; mobile phase: A: water containing 0.1% formic acid; B: containing 0.1% formic acid ACN for formic acid; flow rate: 0.800 mL / min; column temperature: 40 °C; injection volume: 5 mL; UV: monitors 215 nm, 230 nm, 254 nm, 280 nm and 300 nm;
[1371] Gradient table:
[1372] time (minutes) B%
[1373] 0 5
[1374] 2.5 95
[1375] 4...
example 1
[1379] Example 1: 2,4,6-Trichloro-pyrimidine-5-carbaldehyde (Scheme 1)
[1380] After 1.5 hours, to POCl cooled to 0°C 3 (200 mL) in DMF (42 mL) was slowly added barbituric acid (30 g). The mixture was then heated to reflux for 16 hours and then evaporated (disintegrated carefully by pouring the distillate slowly into a stirred slurry of ice methanol). The residue was cooled to 0 °C and added very slowly to the ice-water solution, at which point a beige solid formed. The solid was filtered, dissolved in DCM, washed with water, washed with saturated NaHCO 3 solution washed, dried (MgSO 4 ) and concentrated in vacuo to give white crystals (24g).
example 2
[1381] Example 2: 4,6-Dichloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidine (Scheme 1)
[1382] To a solution of chloroacetaldehyde (3.7 g, 17.5 mmol) dissolved in EtOH (50 mL) and cooled to -78 °C was added methylhydrazine (0.93 mL, 17.5 mmol) and TEA (8 mL). The mixture was stirred at -78°C for 30 minutes, then at 0°C for 2 hours. The solution was then concentrated in vacuo without heating. To the reduced volume solution was added EtOAc and washed with saturated NaHCO 3 Solution The solution was washed and concentrated in vacuo without heating. Filtration through a small plug of silica gel (2:1 EtOAc:Hex) and concentration gave the desired product as a yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com