Anti-infective catheters
An anti-infection and anti-infection agent technology, applied in the direction of catheter, tracheal intubation, biocide, etc., can solve disseminated infection, legacy medical care problems and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0428] Manufacture of Coated Central Venous Catheters
[0429] By using VWR SPEC-WIPE saturated with 75 / 25 IPA / MEK The 7 wiper wiped and cleaned the CVC from the proximal end to the distal end of the CVC body. Catheters were dried at ambient temperature for a minimum of 60 minutes.
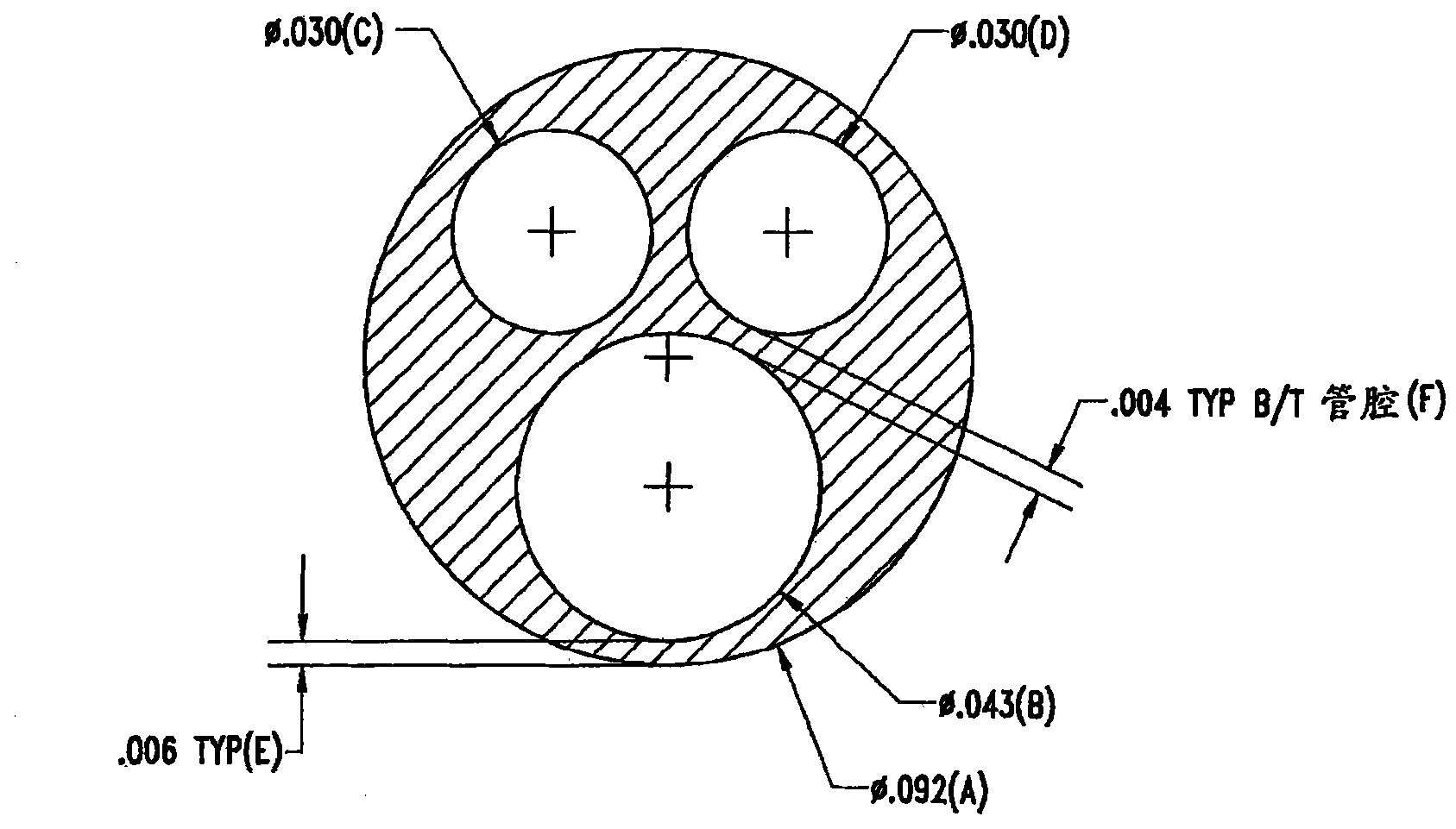
[0430] The conduits were then mounted on angle iron brackets used as coating jigs. Place the applicator cup on the conduit and install the angle iron bracket on the applicator. The coating solution prepared according to the present invention was added to the coating cup and the catheter was coated. During this procedure, the lumen of the catheter is purged of air to ensure that the lumen and ports are not entrapped with coating solution.
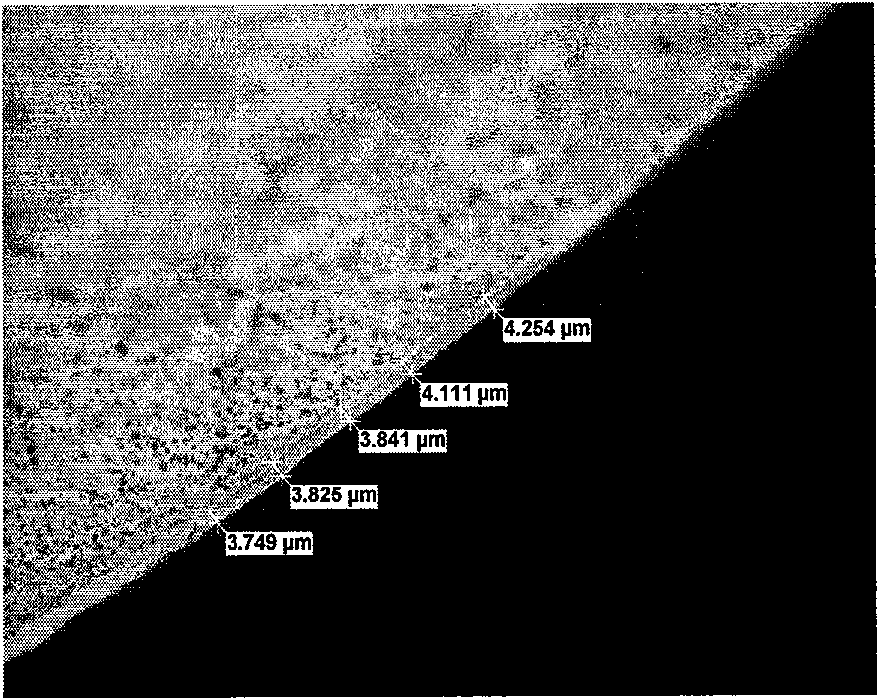
[0431] Take the coated catheter out of the coating machine, and dry it in a ventilated oven at 85±5°C for 20 minutes to remove residual solvents to a qualified level. The coated catheter was removed from the oven and allowed to cool. The coated catheters ...
Embodiment 2
[0436] Drug Elution from Coated Catheters
[0437] The in vitro elution profile of 5-FU from CVC coatings prepared as described in Example 1 was determined. Elution was performed by immersing a 4 cm portion of the coated catheter sample in 15 mL of phosphate buffered saline, pH 7.4 (PBS) at 37°C. Place the sample in a rotating device for stirring. Elution medium was sampled at selected times and analyzed by HPLC. Such as image 3 As shown, 5-FU gradually eluted from the catheter coating, releasing nearly 50% of the drug on the seventh day and 90% on the twenty-eighth day.
Embodiment 3
[0439] Stability of Coated Catheters
[0440] A stability study was performed using coated catheters manufactured according to Example 1 to determine the shelf life / expiration date of these catheters. Experimental evaluation of drug components includes drug identity, drug content, and in vitro elution. Evaluation of the coated polymers included visual inspection, dry adhesion and wet abrasion / wet peel tests.
[0441]Catheters were tested for stability using both real-time (25°C / 60% RH) and accelerated (40°C / 75% RH) conditions. Based on the analysis of the data, at 24 months at 25°C and 60% RH, with a 95% confidence level, (1) the total 5-FU content of the catheter is not expected to fall below 92.92% of the initial value, (2) The purity of the drug is expected to remain above 95.25%, (3) The defect rate in the dry adhesion and wet peel / wet abrasion tests of the coating is expected to be less than 5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com