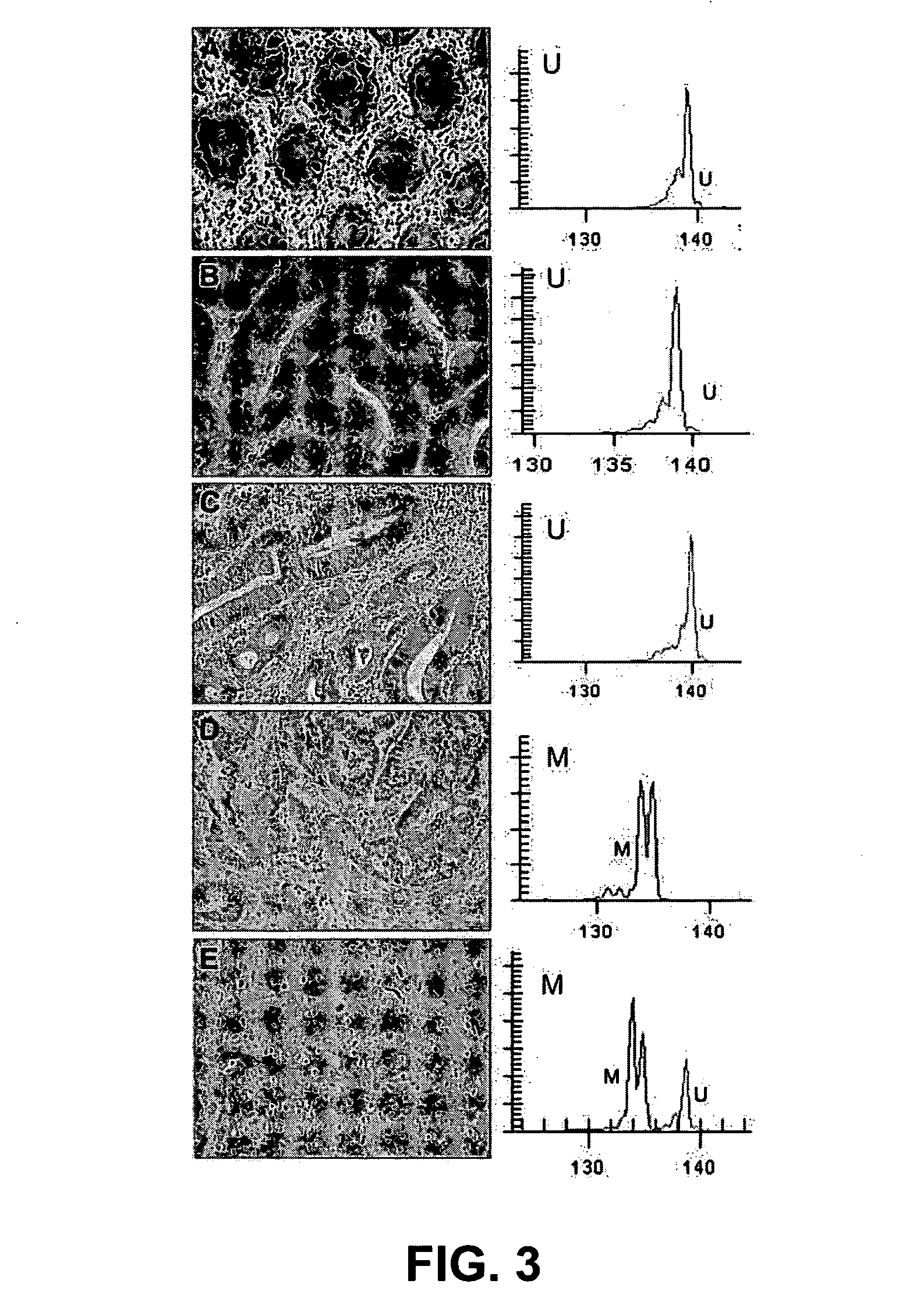
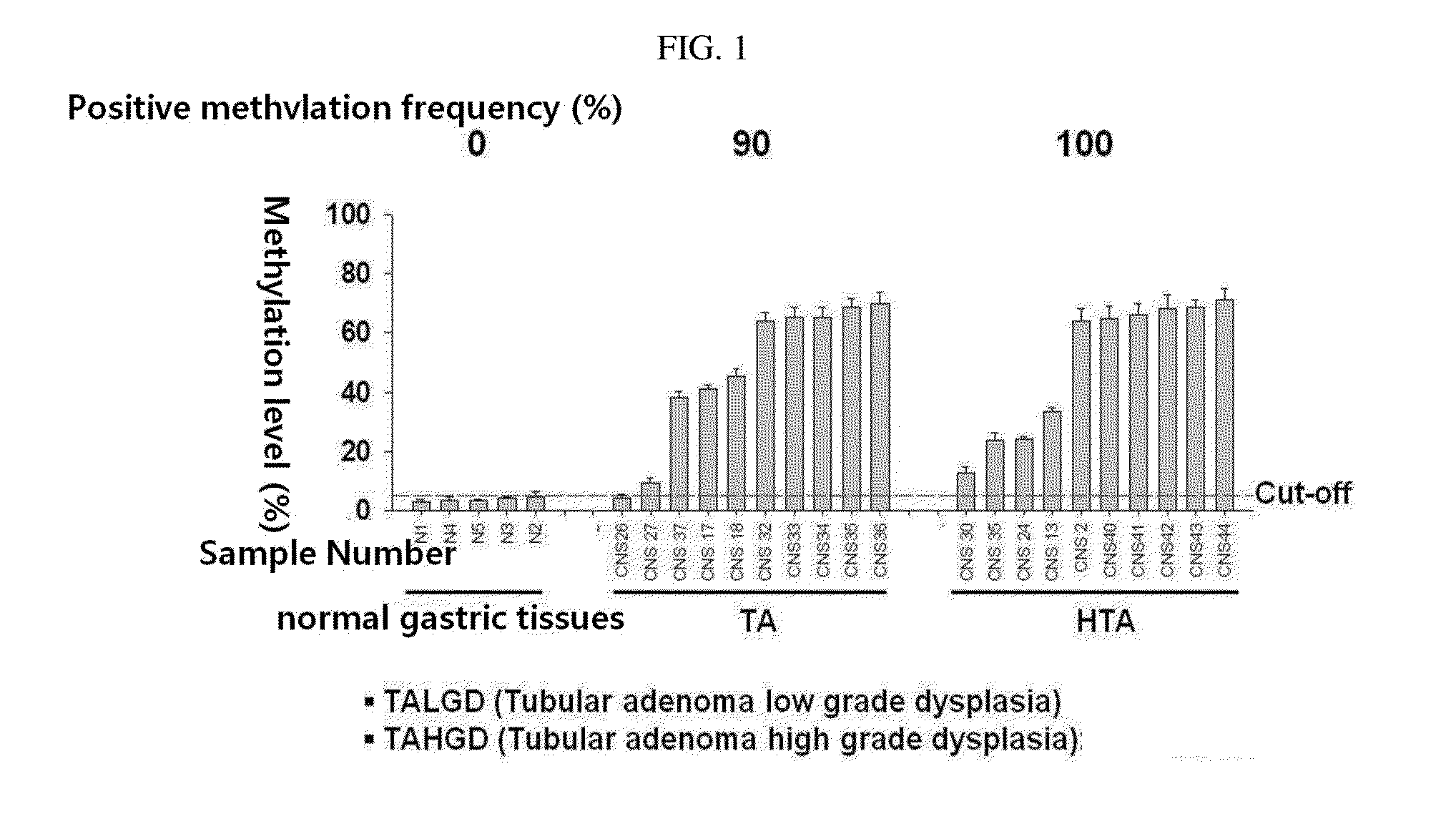
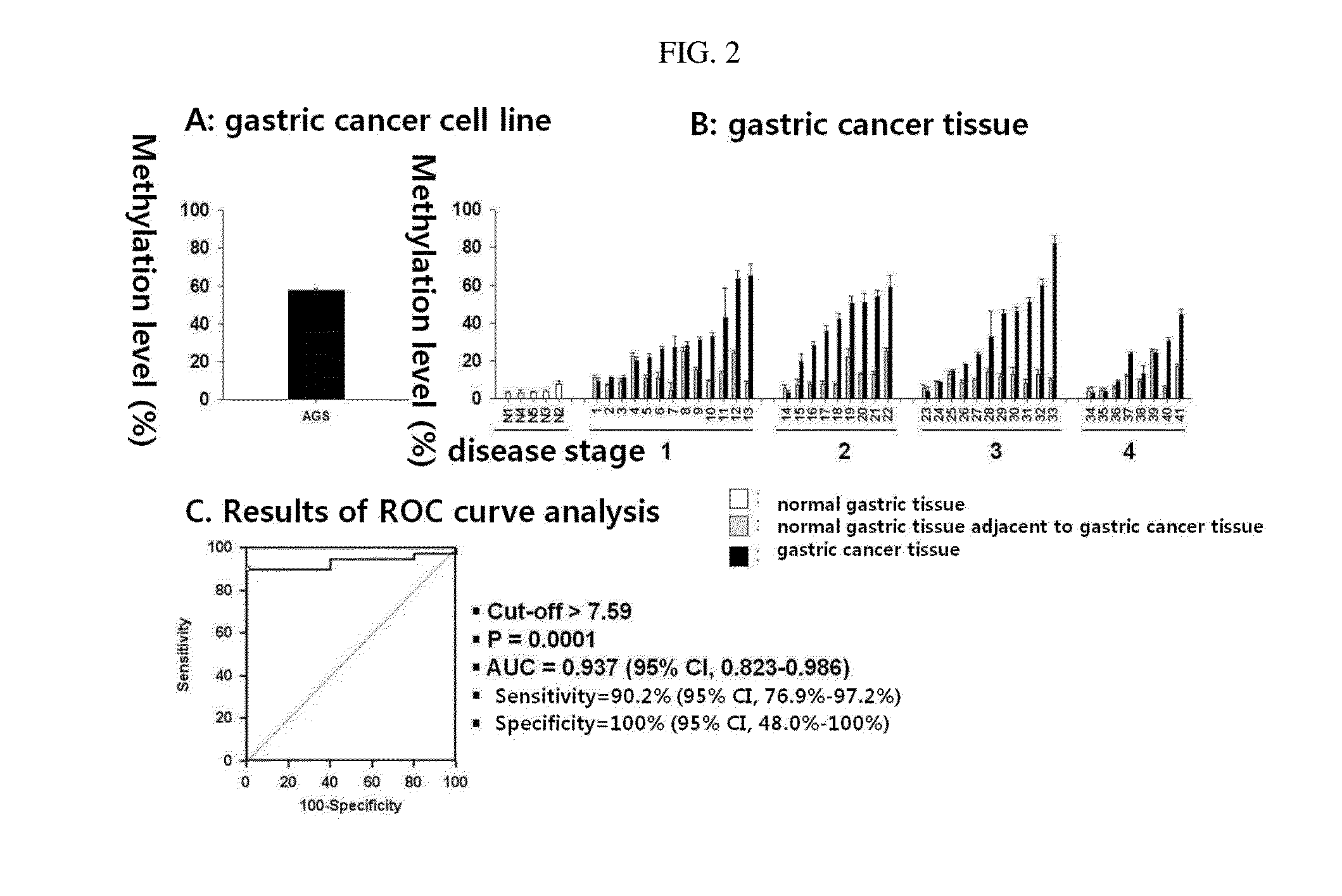
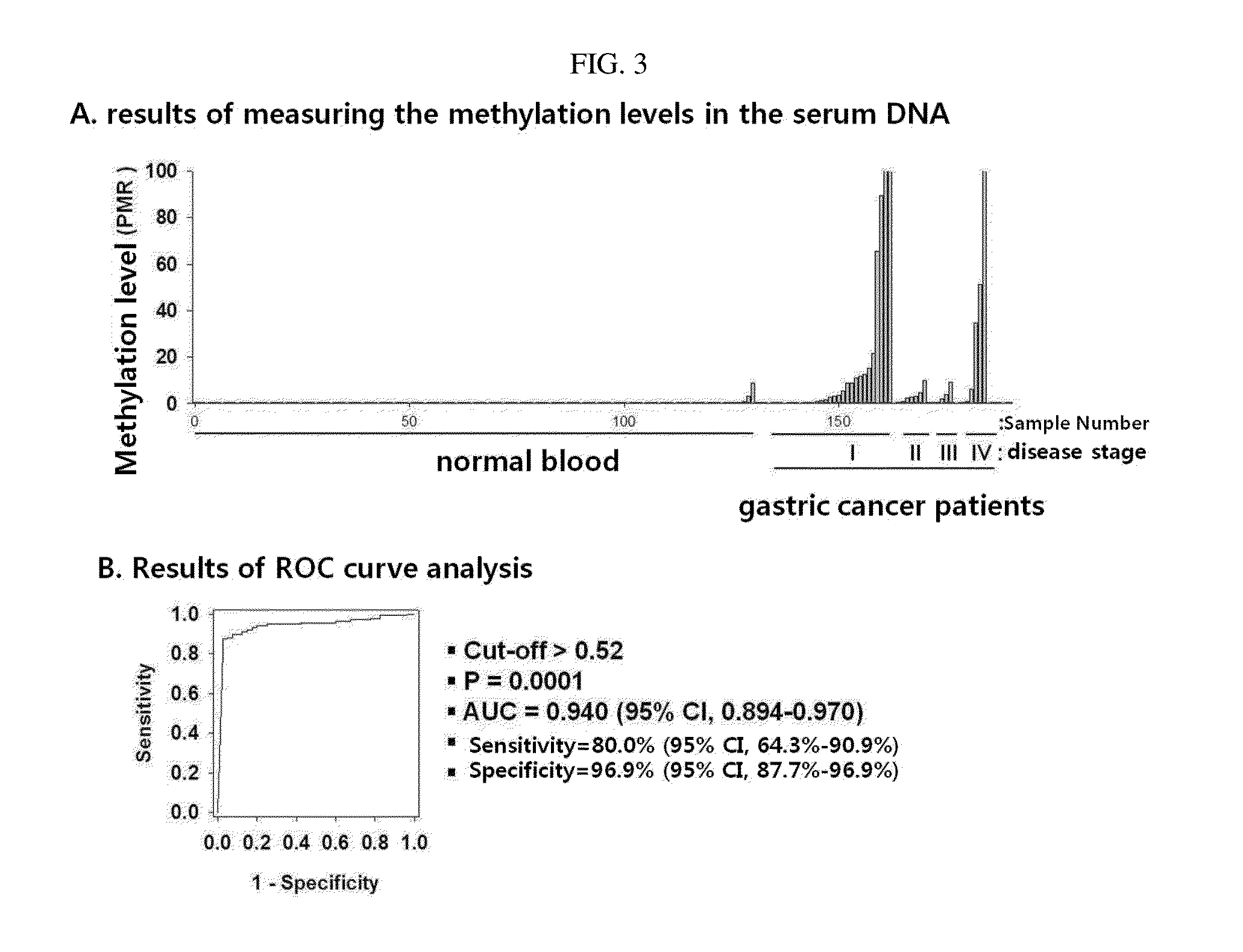
Patents
Literature
145 results about "Mgmt methylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
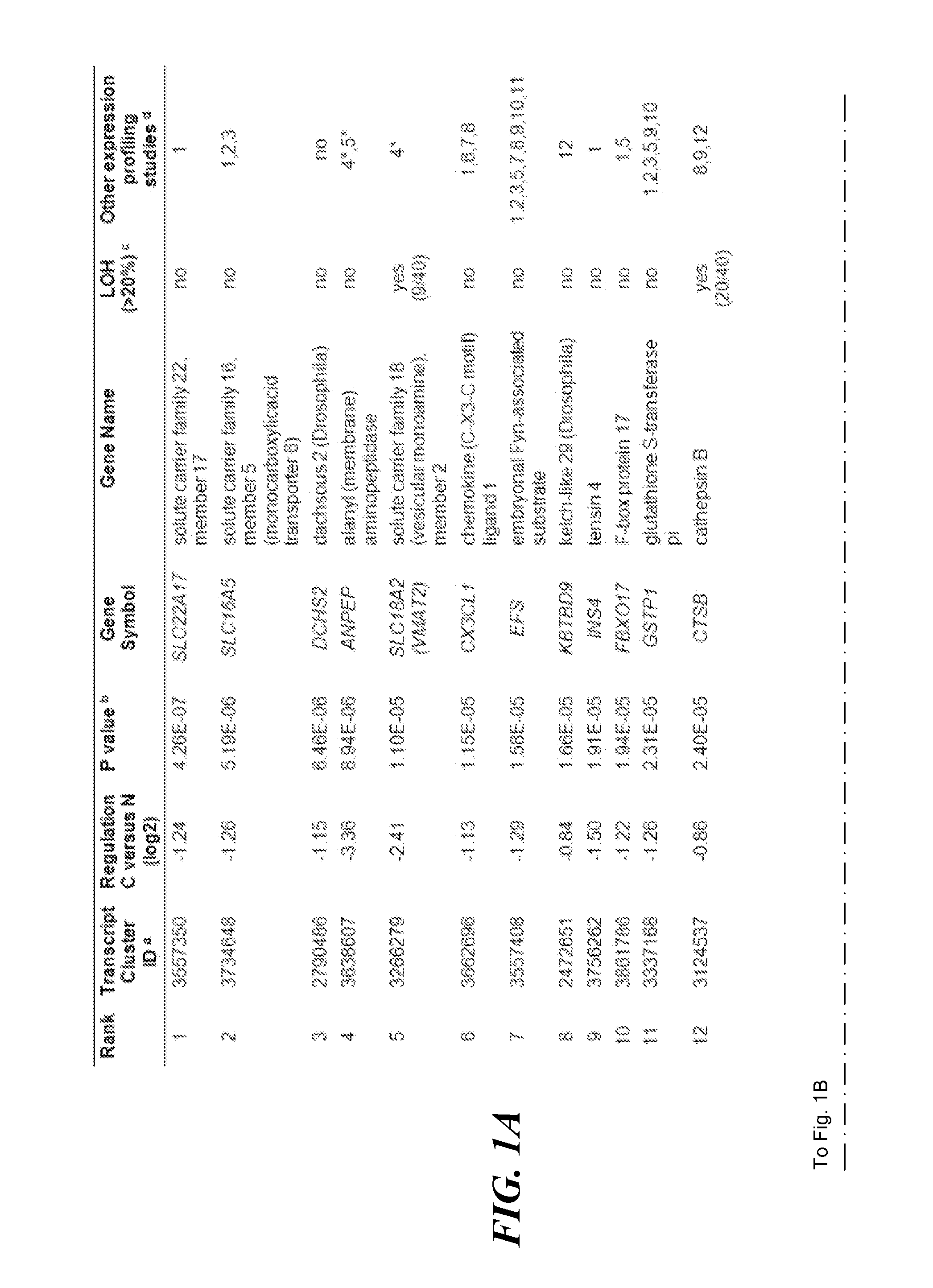
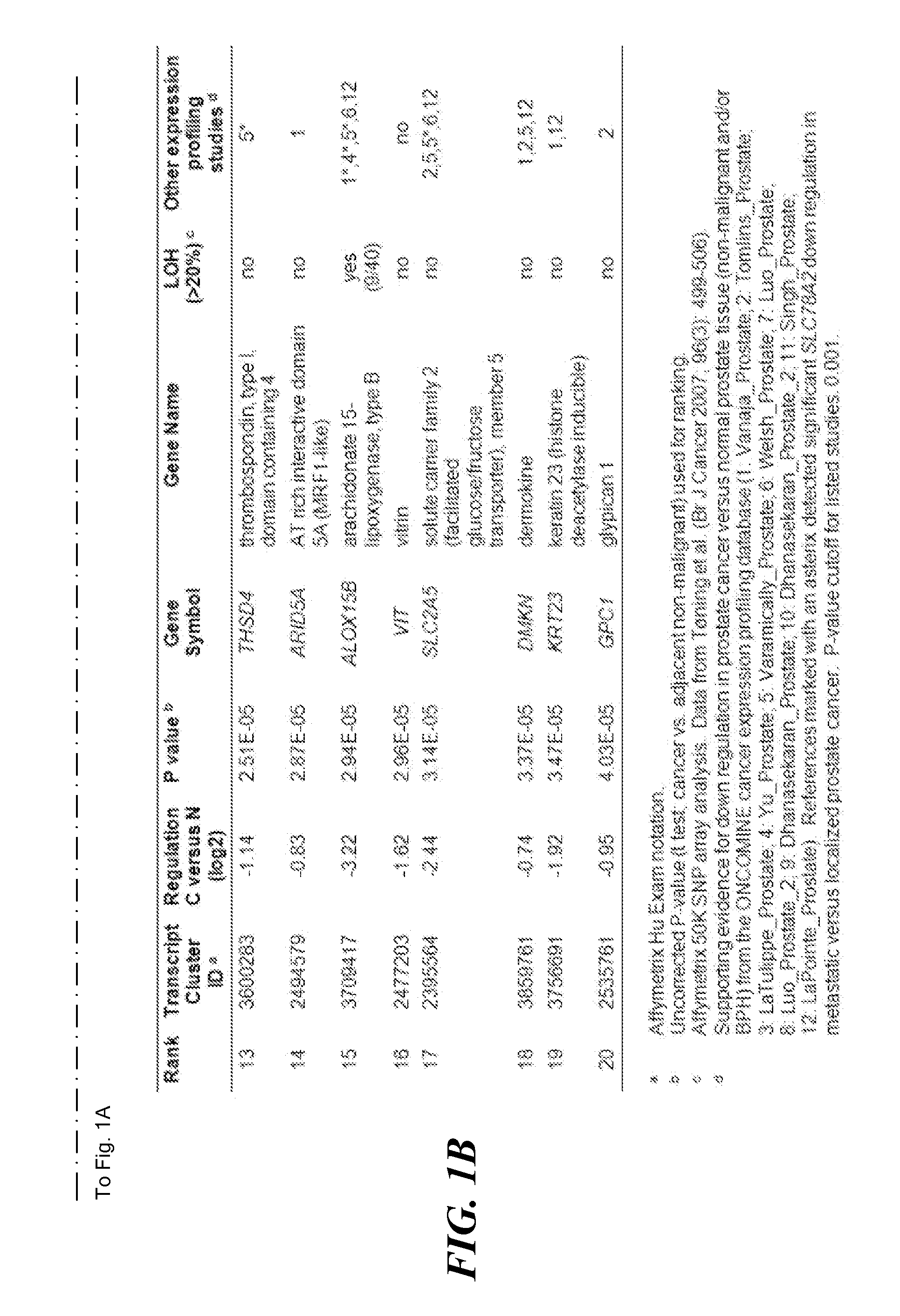
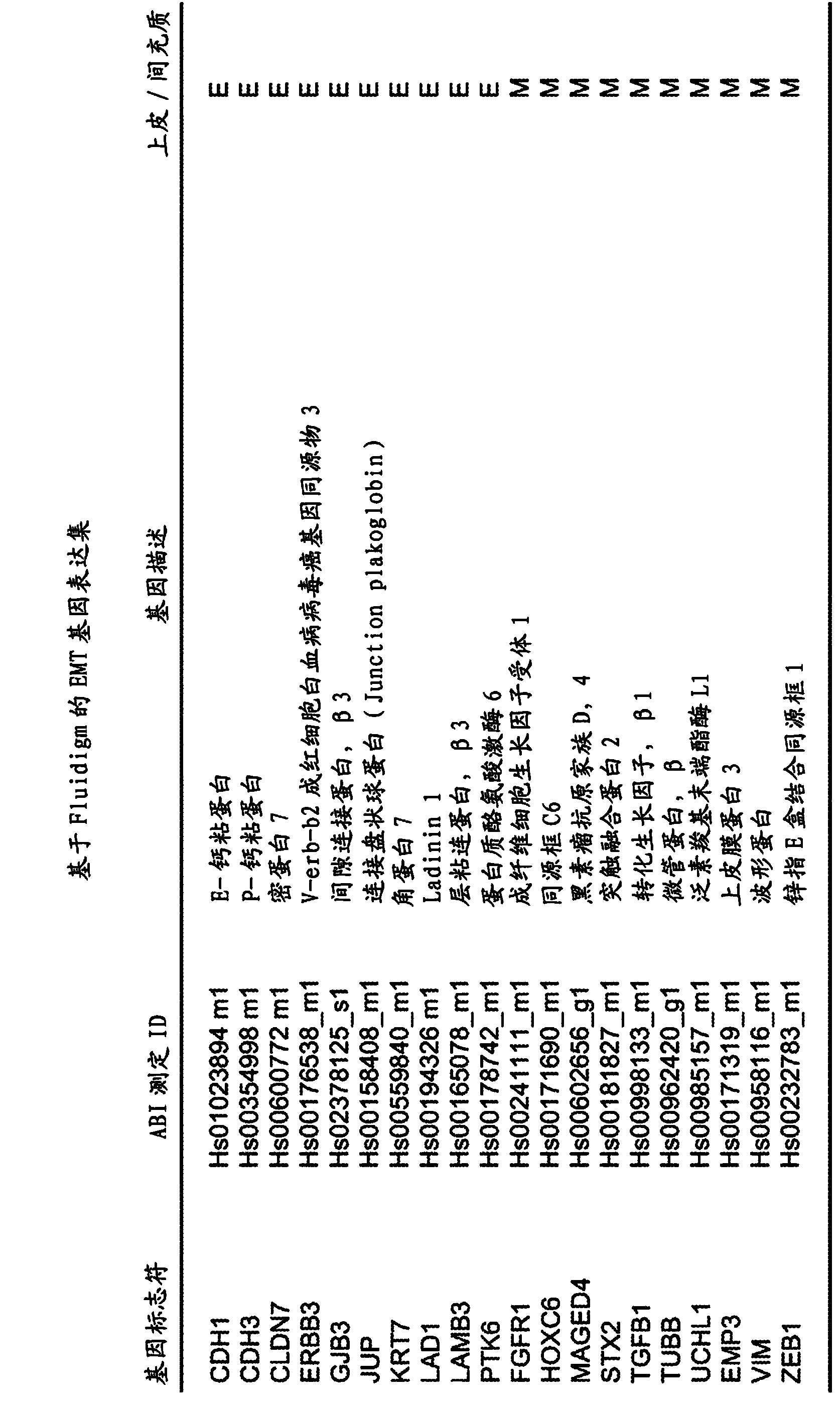
MGMT Methylated. MGMT methylated is when the genetics responsible for a type of brain tumor called a glioblastoma have been altered by a chemical process called methylation. A MGMT methylated glioblastoma allows for a better patient response to treatments, specifically temozolomide and radiation.
Compositions and methods for reestablishing gene transcription through inhibition of DNA methylation and histone deacetylase
InactiveUS6905669B2Better clinical outcomeReduce dosageBiocideCell receptors/surface-antigens/surface-determinantsCyclic peptideDisease
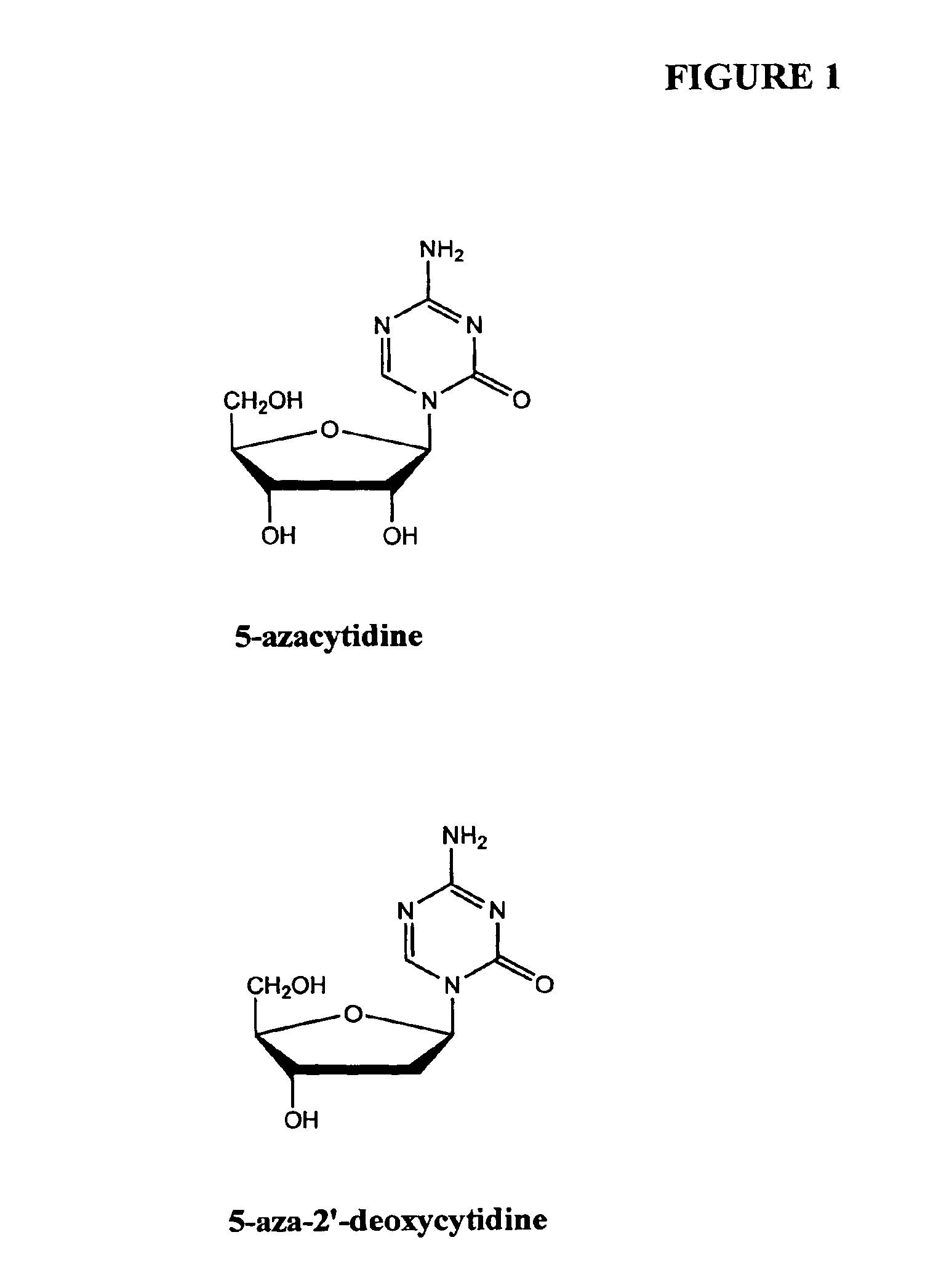
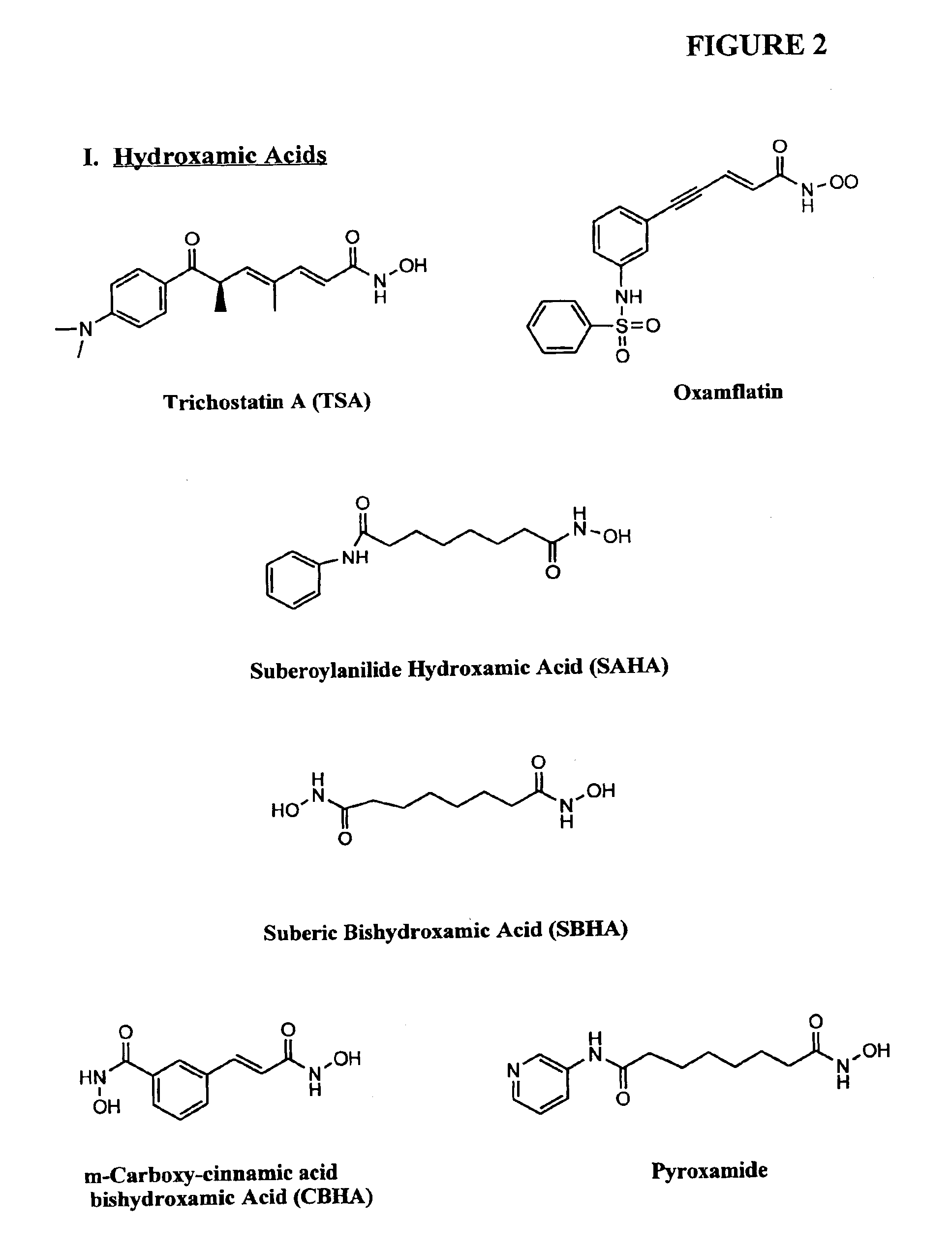
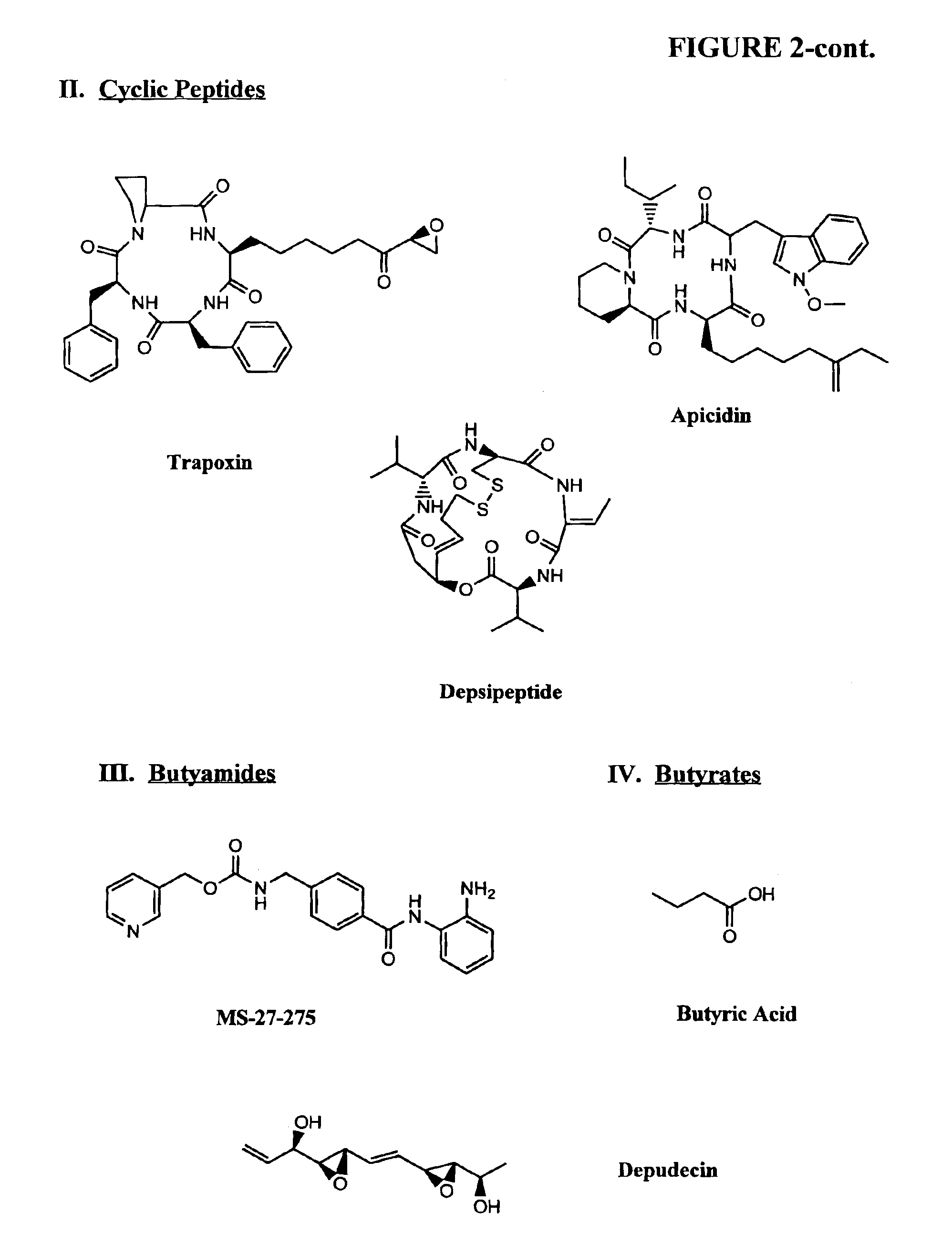
Compositions and methods are provided for treating diseases associated with aberrant silencing of gene expression such as cancer by reestablishing the gene expression through inhibition of DNA hypomethylation and histone deacetylase. The method comprises: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
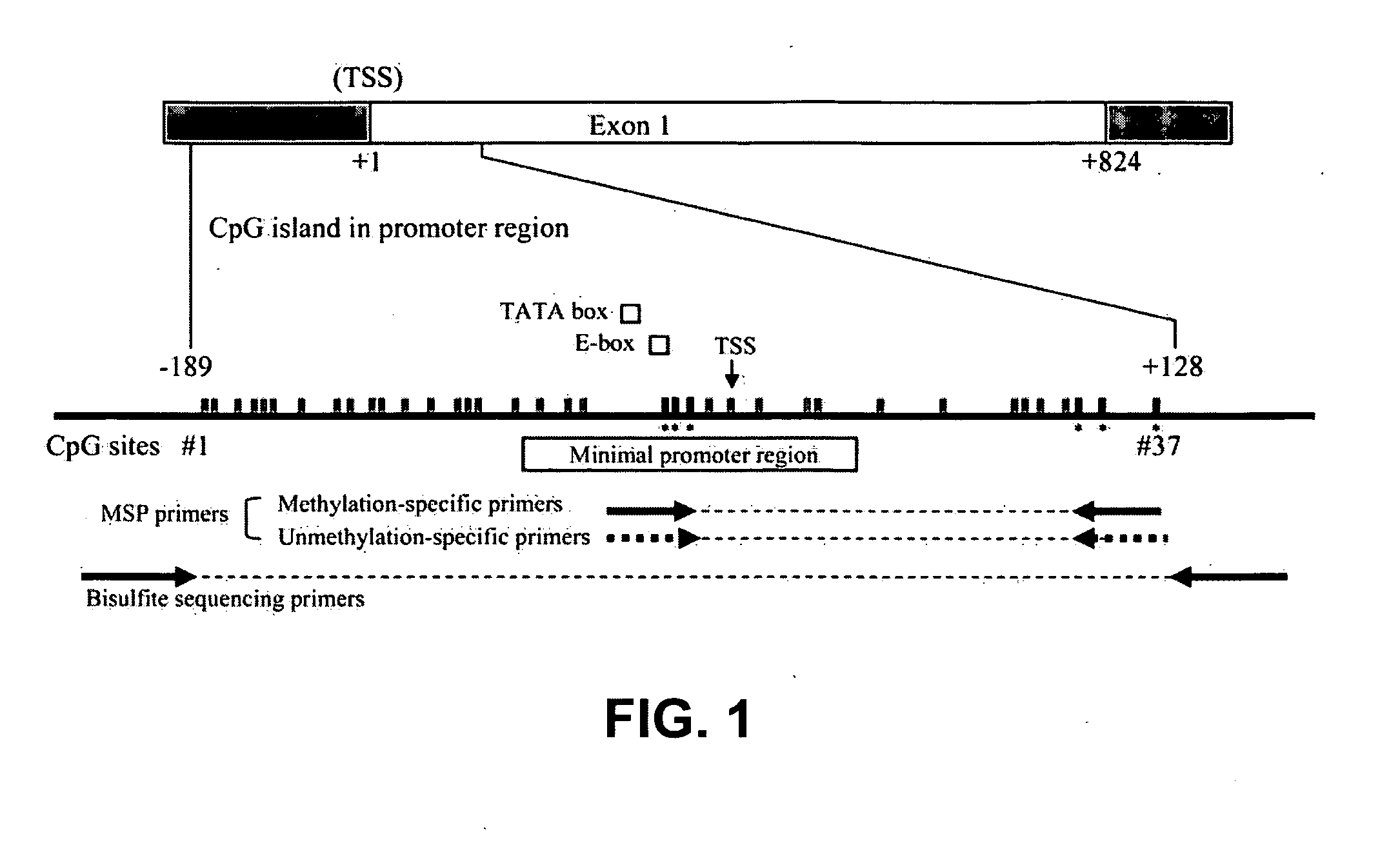
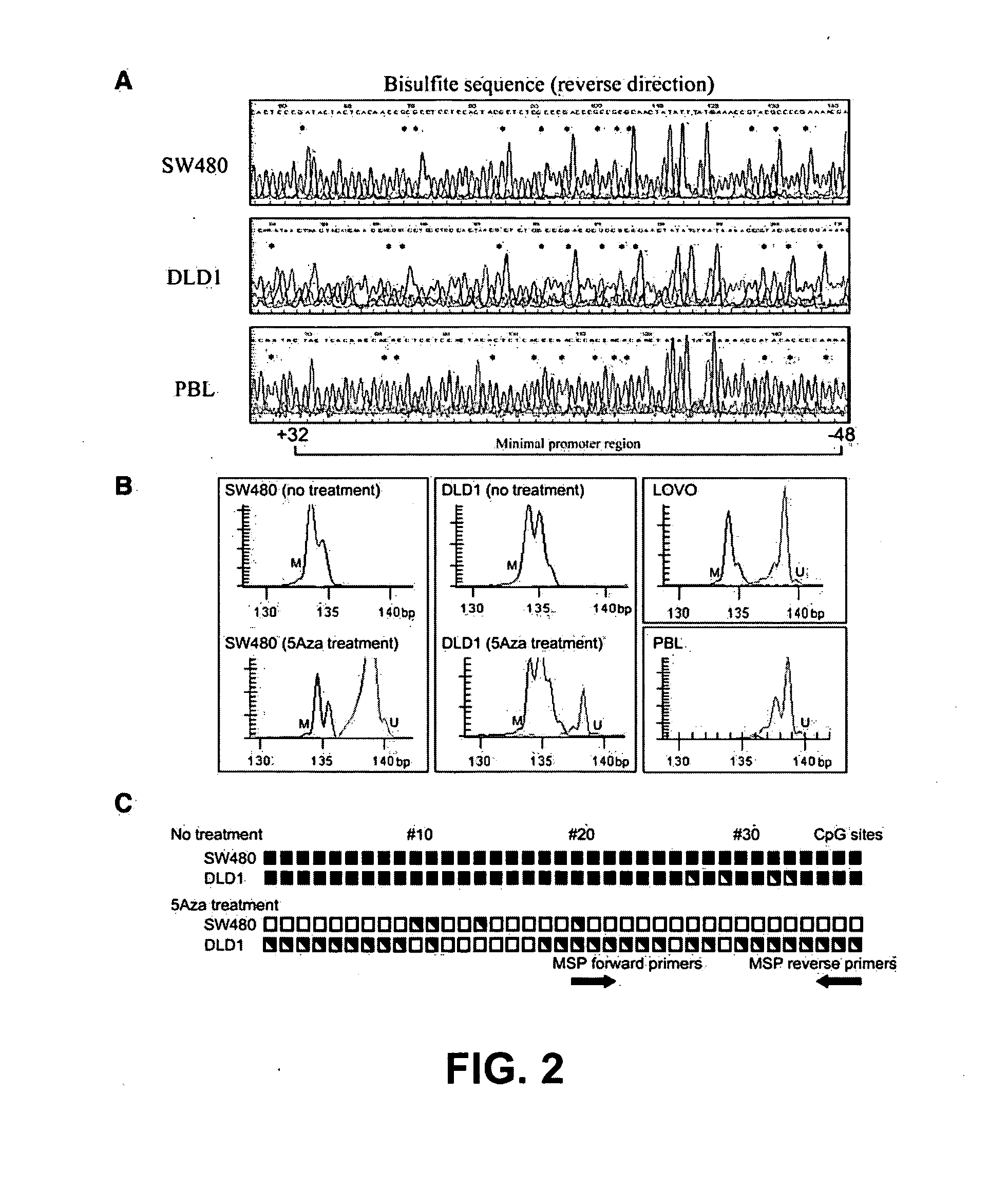
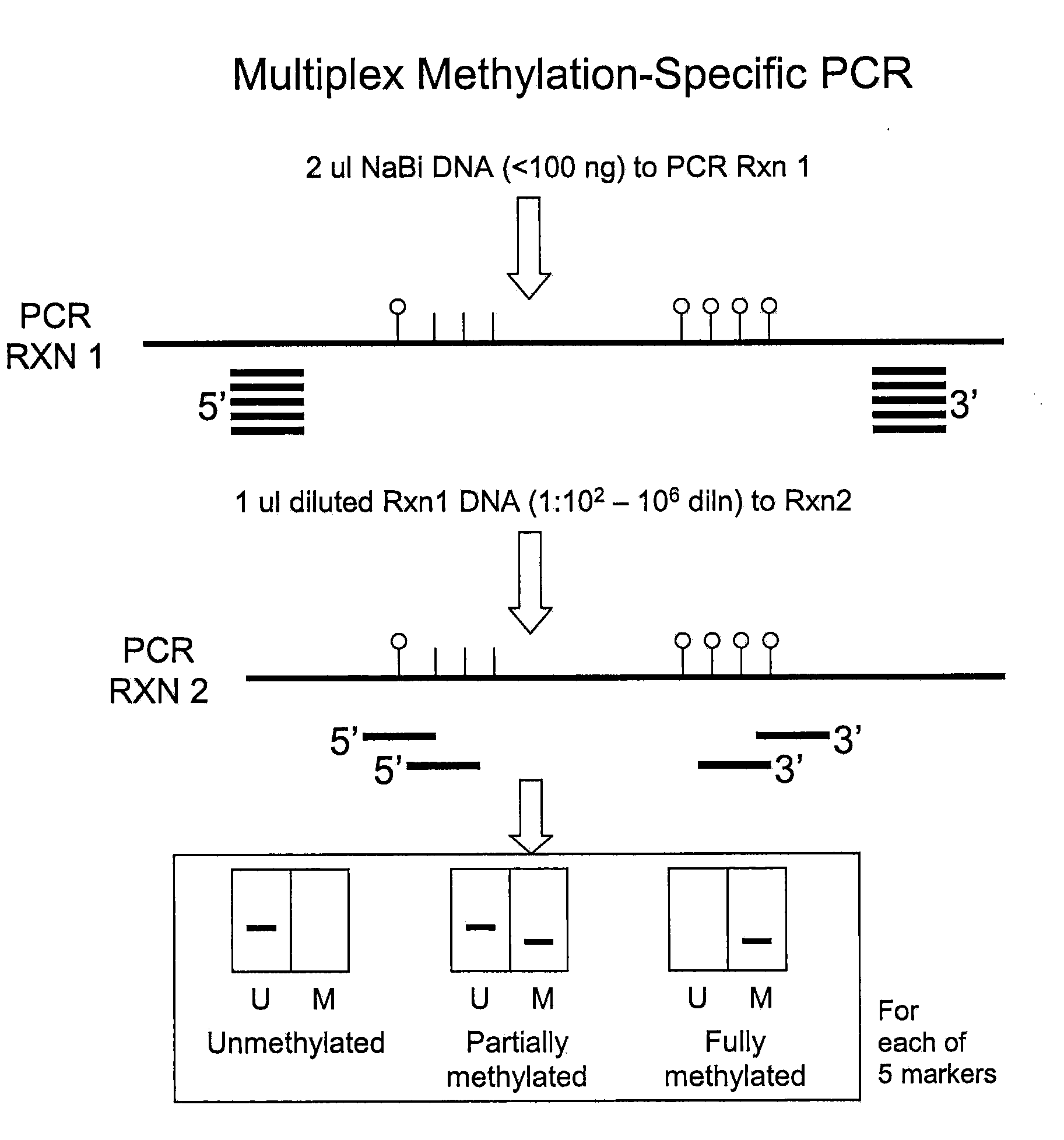
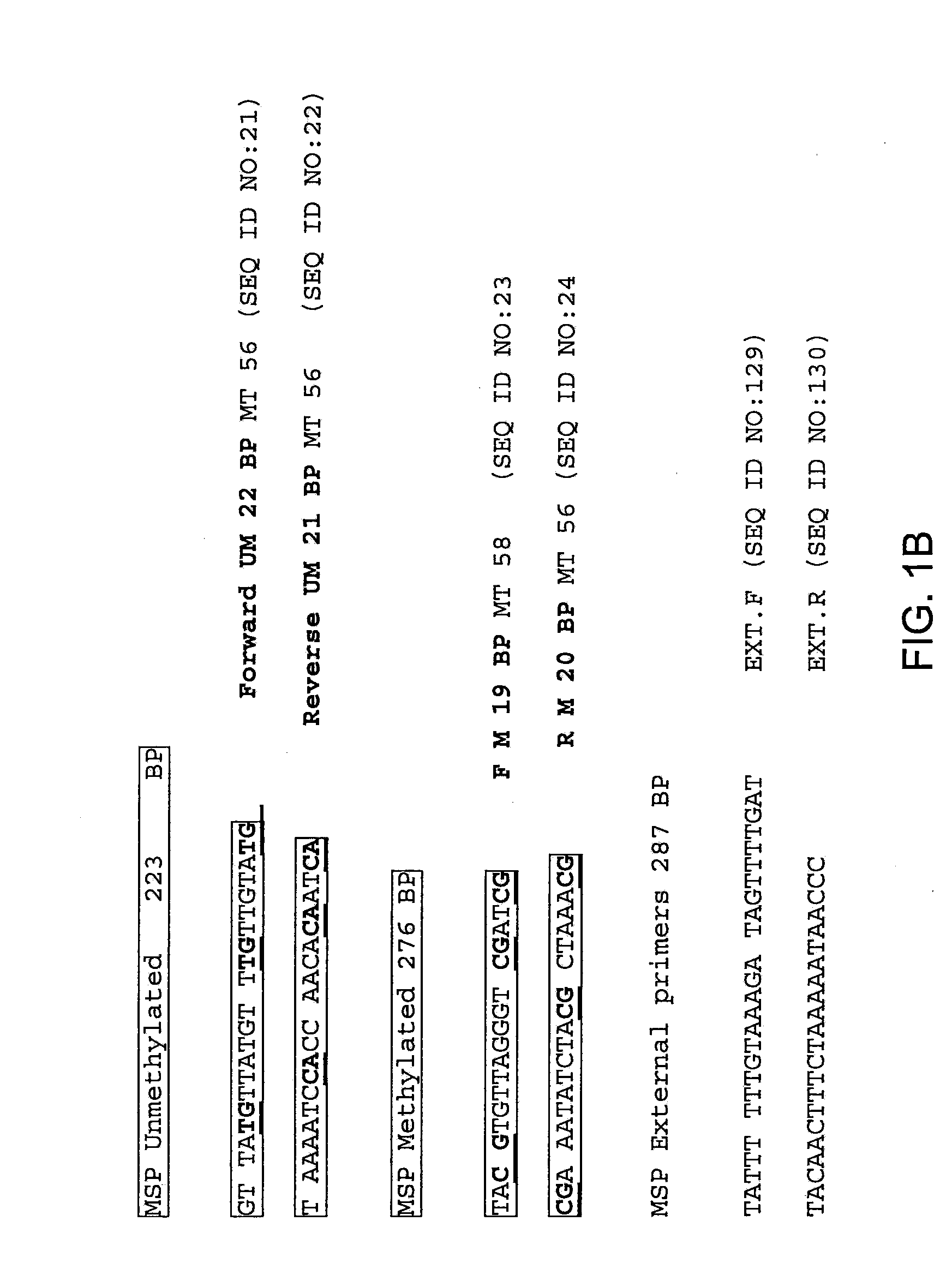
Nested methylation-specific polymerase chain reaction cancer detection method
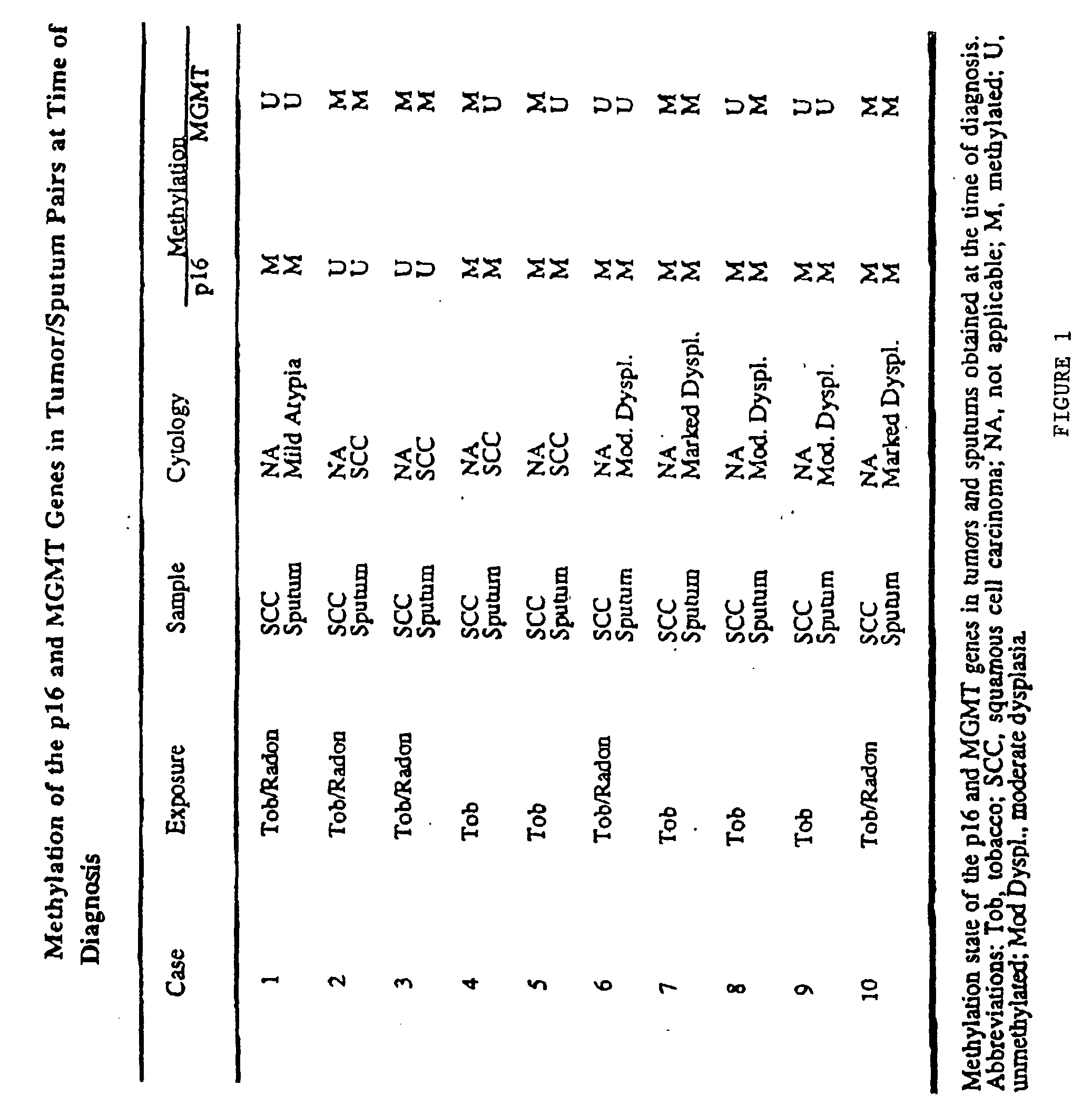
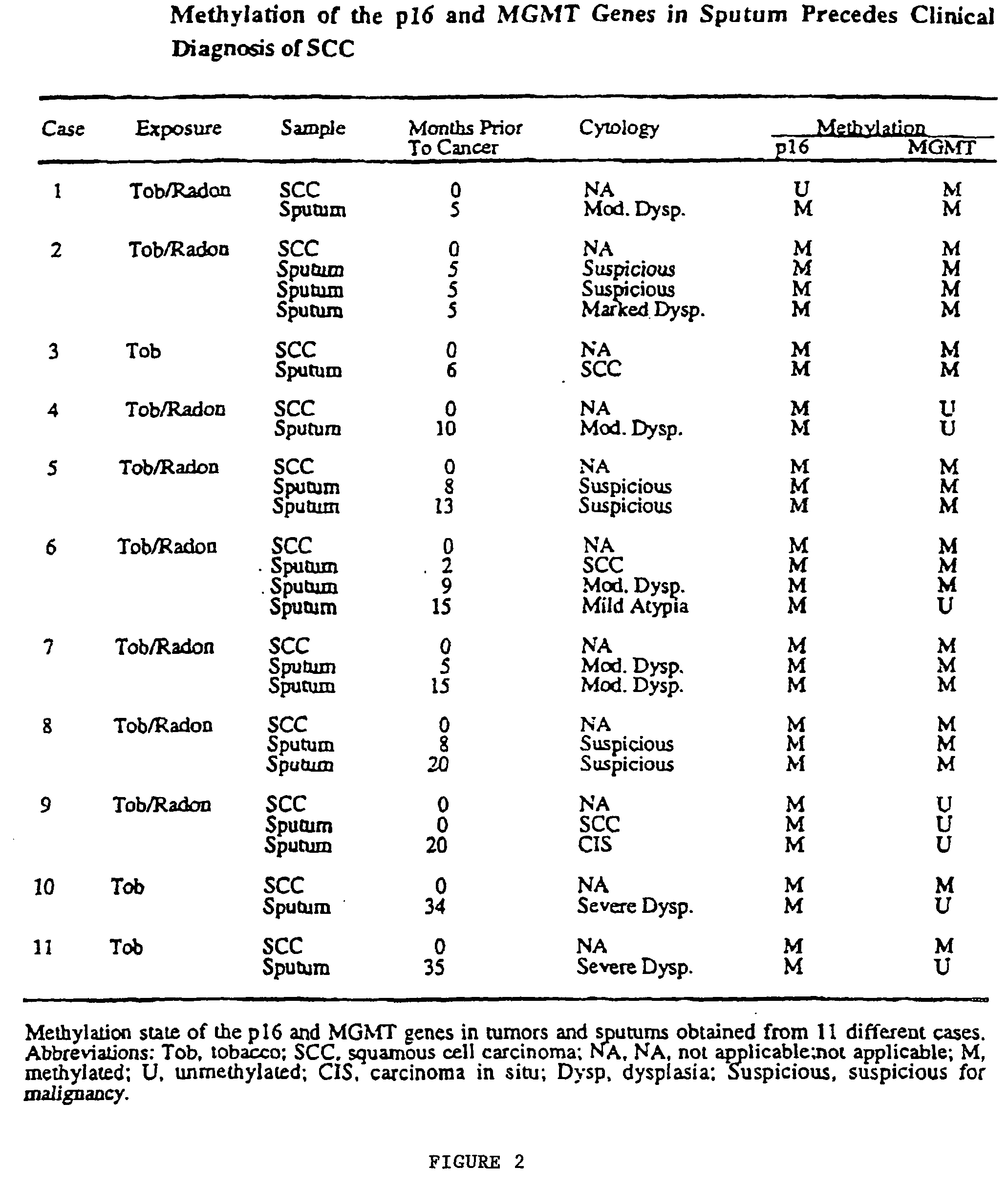
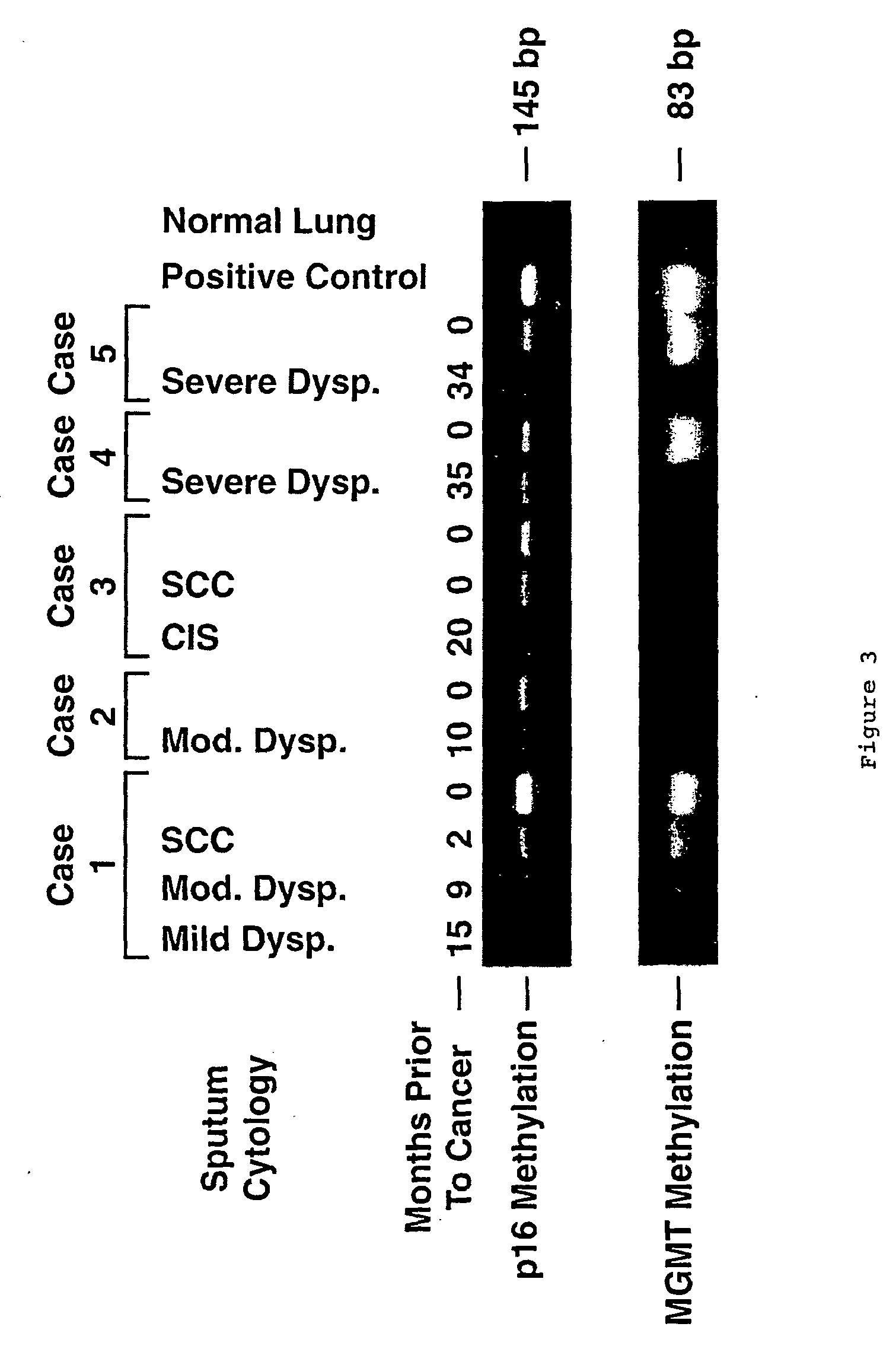
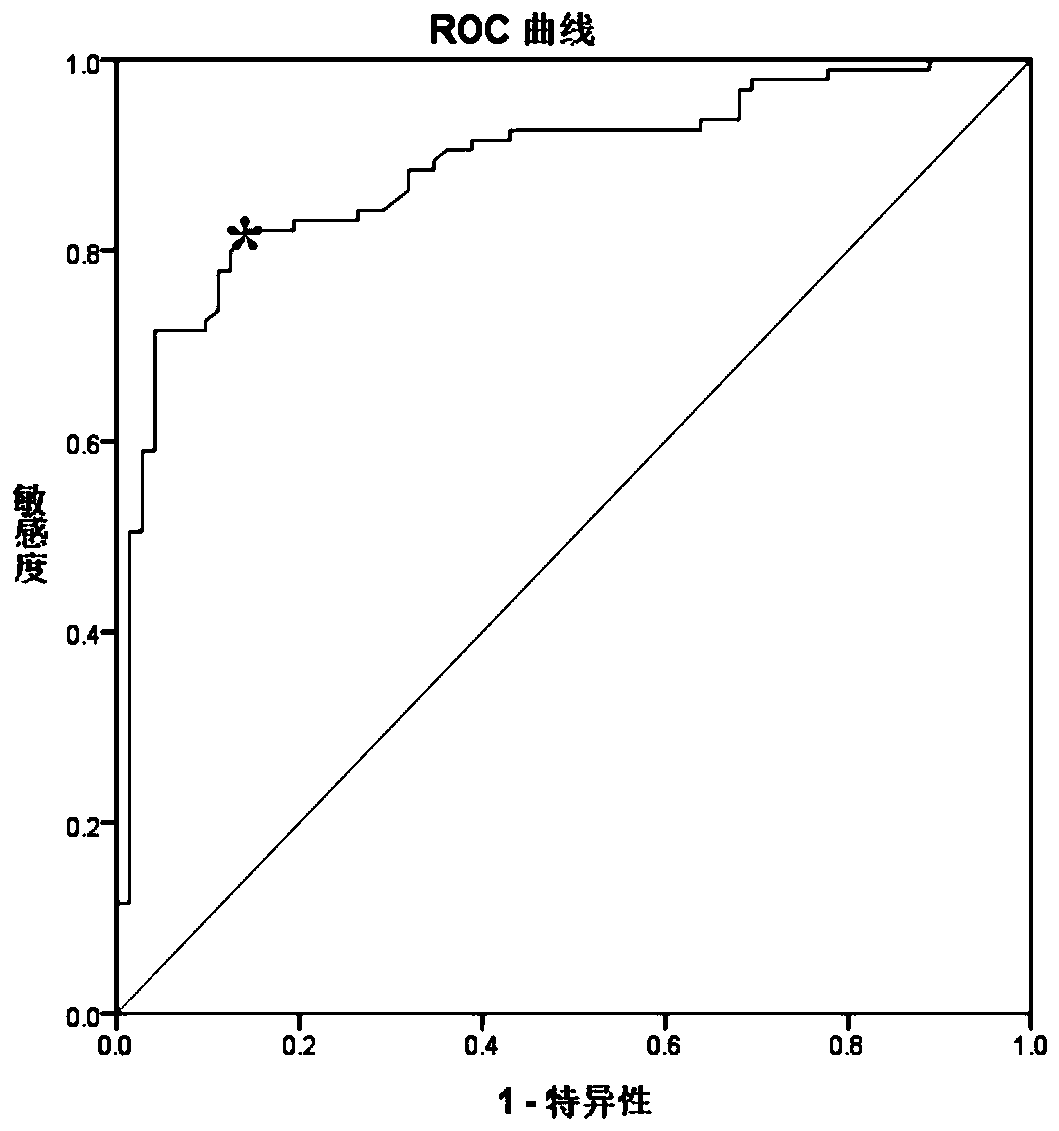
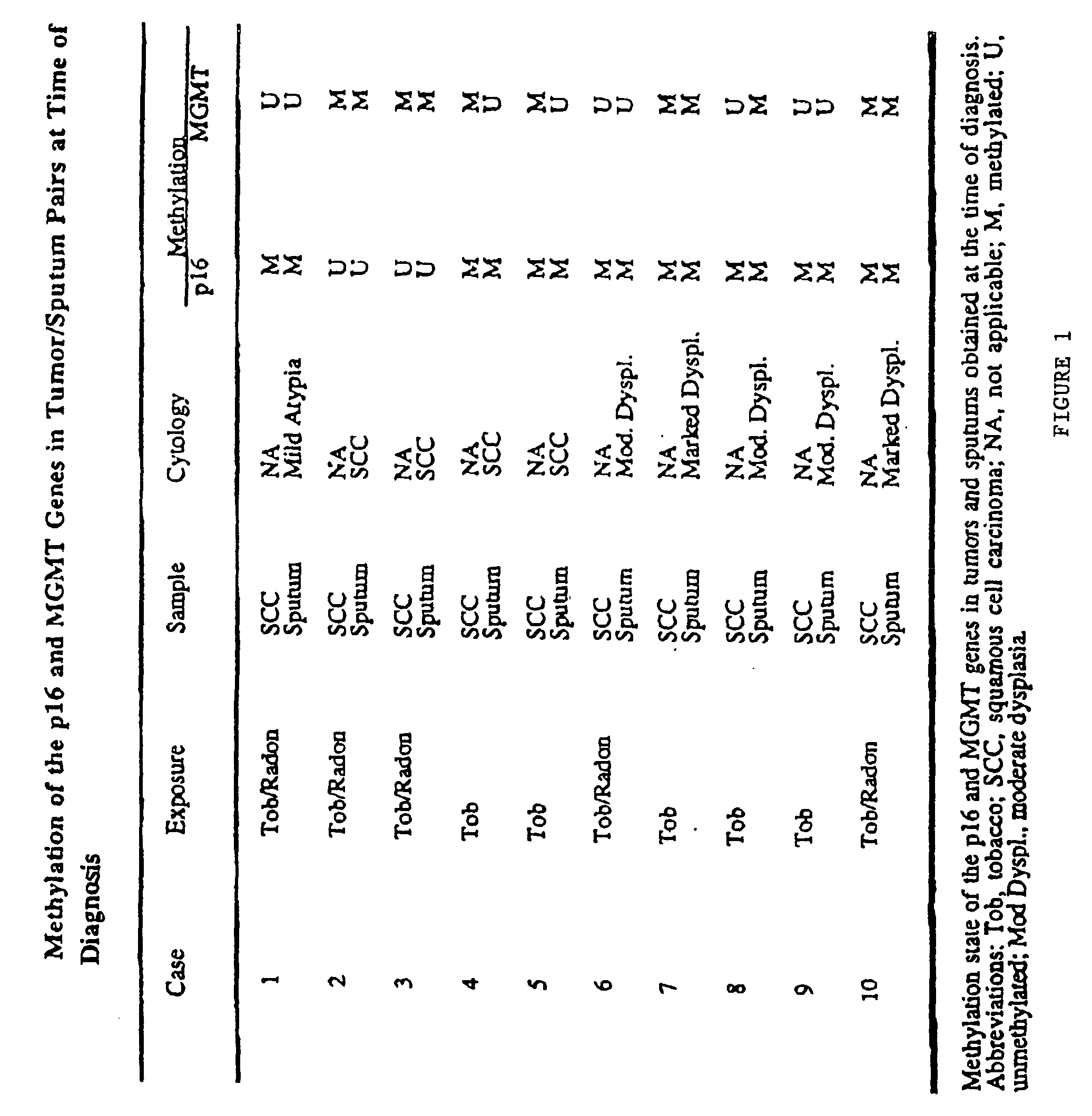
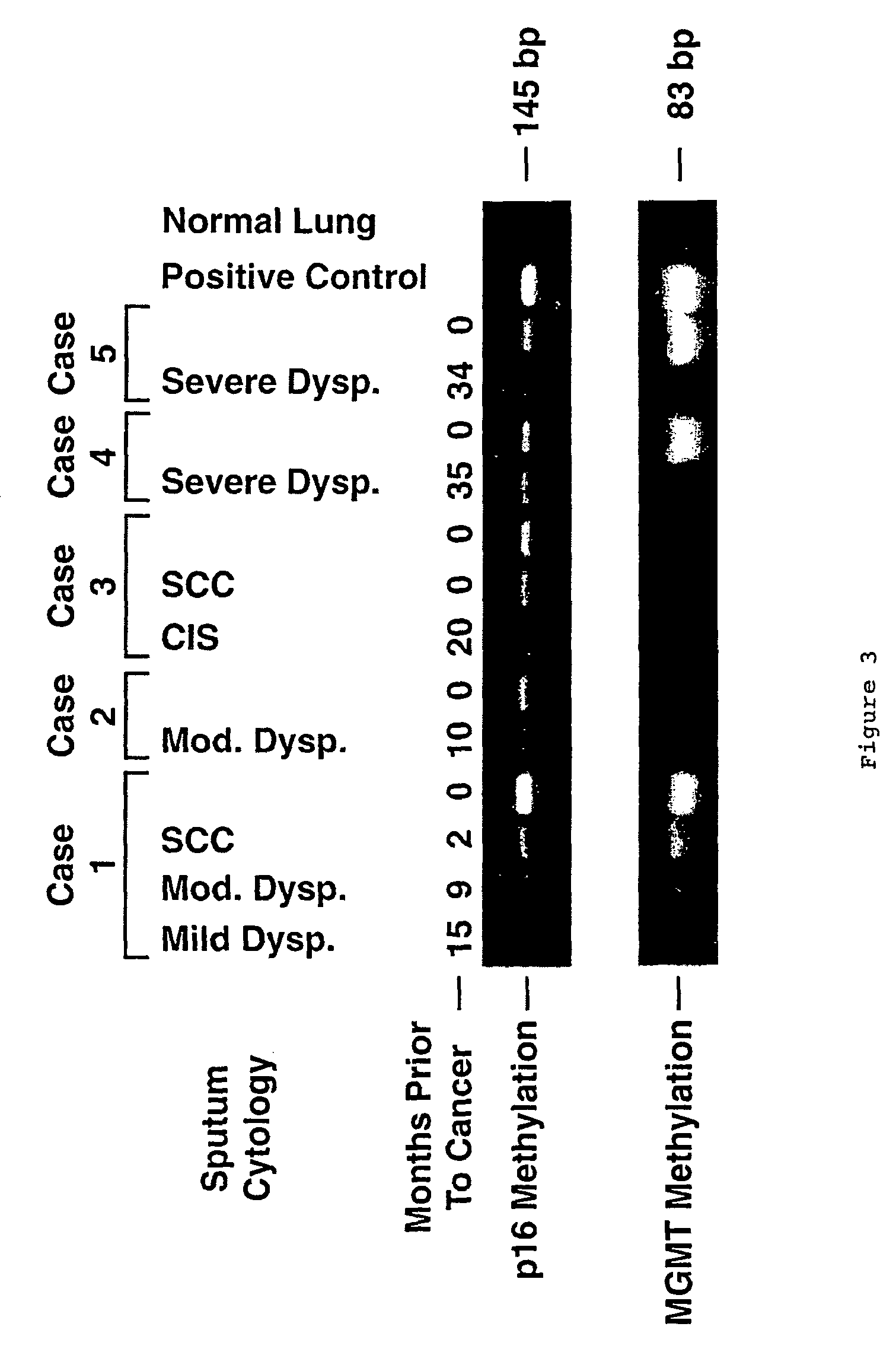
A molecular marker-based method for monitoring and detecting cancer in humans. Aberrant methylation of gene promoters is a marker for cancer risk in humans. A two-stage, or "nested" polymerase chain reaction method is disclosed for detecting methylated DNA sequences at sufficiently high levels of sensitivity to permit cancer screening in biological fluid samples, such as sputum, obtained non-invasively. The method is for detecting the aberrant methylation of the p16 gene, O 6-methylguanine-DNA methyltransferase gene, Death-associated protein kinase gene, RAS-associated family 1 gene, or other gene promoters. The method offers a potentially powerful approach to population-based screening for the detection of lung and other cancers.
Owner:LOVELACE RESPIRATORY RES INST
Early detection and prognosis of colon cancers
InactiveCN101688239ASugar derivativesMicrobiological testing/measurementGenes mutationTumor specific
We have developed a transcriptome-wide approach to identify genes affected by promoter CpG island hypermethylation and transcriptional silencing in colorectal cancer (CRC). By screening cell lines andvalidating tumor specific hypermethylation in a panel of primary human CRC samples, we estimate that nearly 5% of all known genes may be promoter methylated in an individual tumor. When directly compared to gene mutations, we find a much larger number of genes hypermethylated in individual tumors, and much higher frequency of hypermethylation within individual genes harboring either genetic or epigenetic changes. Thus, to enumerate the full spectrum of alterations in the human cancer genome, and facilitate the most efficacious grouping of tumors to identify cancer biomarkers and tailor therapeutic approaches, both genetic and epigenetic screens should be undertaken. The genes we identified can be used inter alia diagnostically to detect cancer, pre-cancer, and likelihood of developing cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
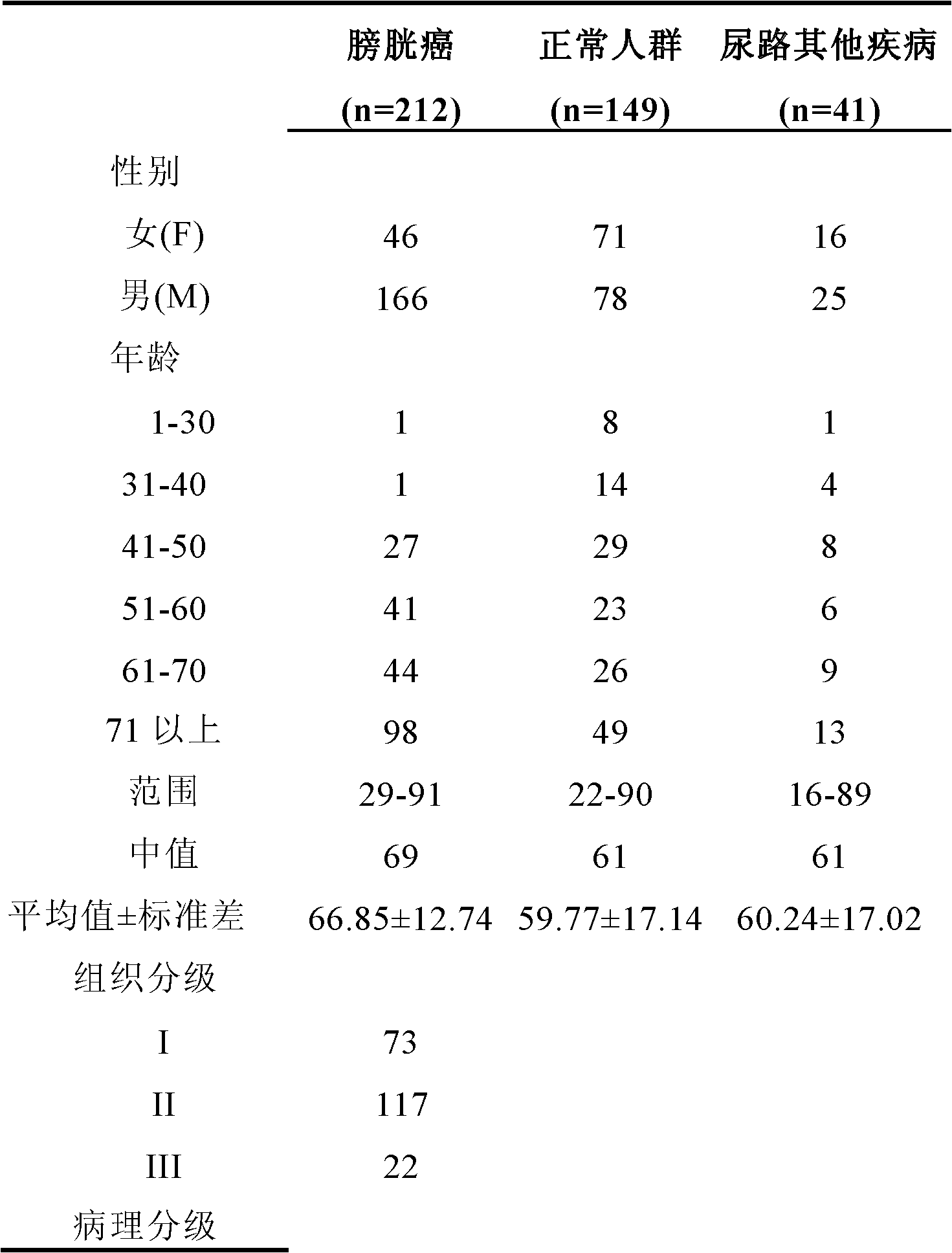
Method and kit for diagnosing bladder cancer with urine
InactiveCN102311953AMicrobiological testing/measurementDNA/RNA fragmentationCommunity health centerBiomarker (petroleum)
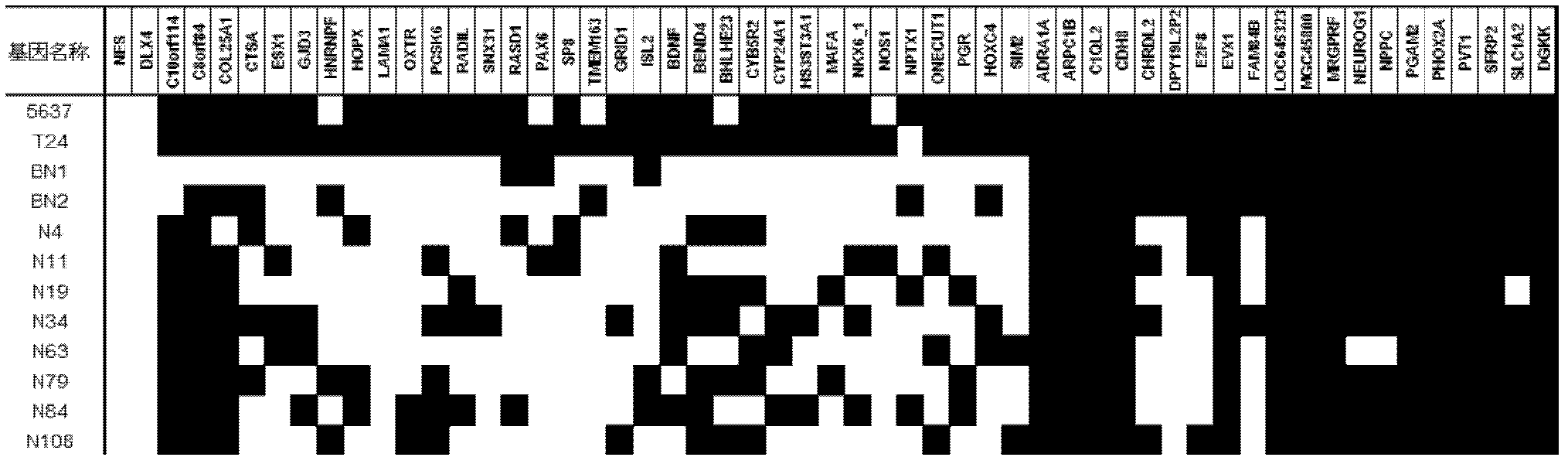
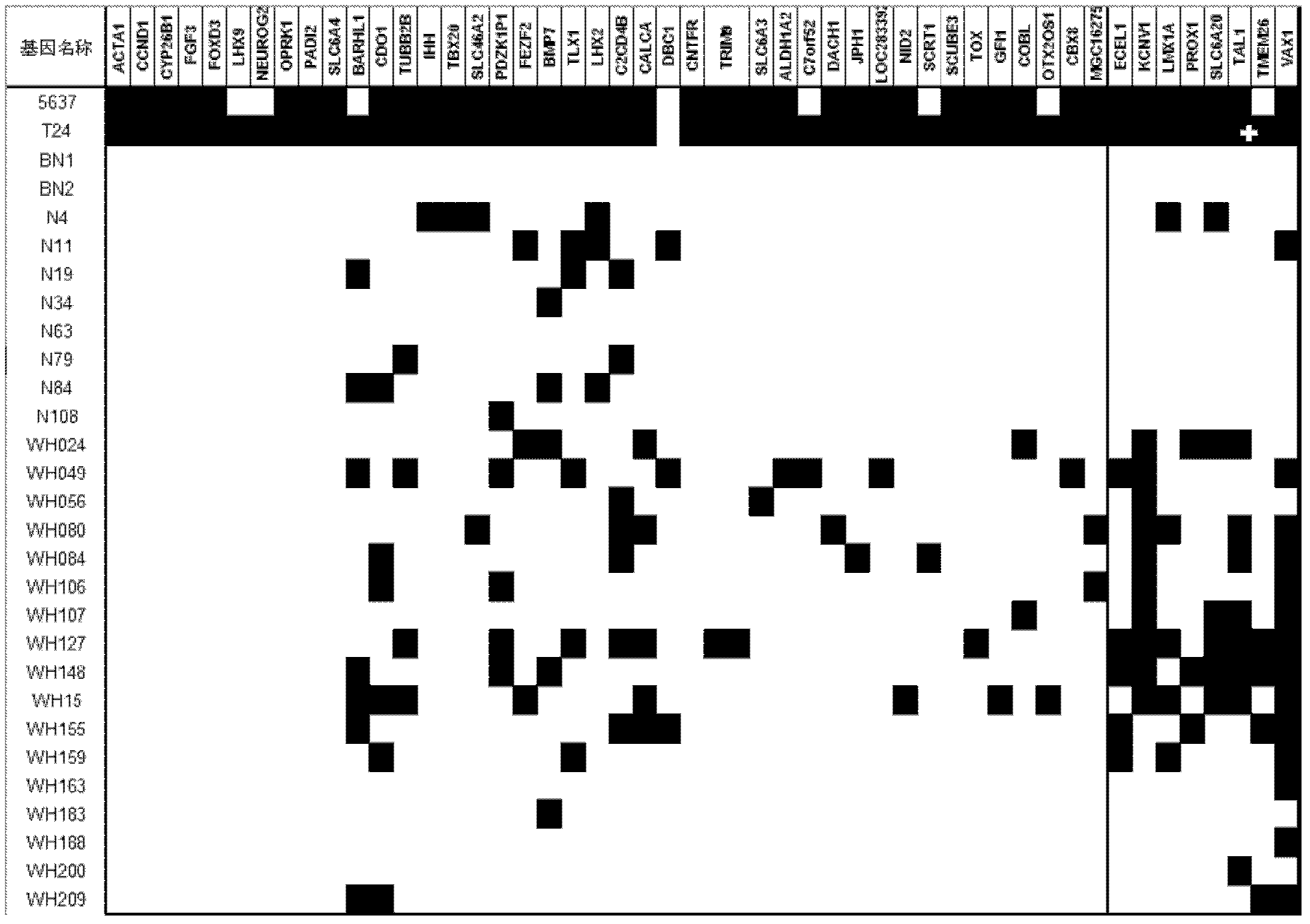
The invention relates to a method and a kit for diagnosing bladder cancer with urine. The invention discloses a group of methylated sensitive genes, comprising ECEL1, KCNV1, LMX1A, PROX1, SLC6A20, TAL1, TMEM 26, and VAXI gene. In urine samples of bladder cancer patients, the specific CpG sites of the genes are showed the highest methylation levels. Accordingly, the genes are the biological marker of bladder cancer. The method and the kit can be used as the basic of designing diagnostic reagents of bladder cancer, and are suitable for auxiliary detection of cancer in hospitals, postoperative followup, screening of high risk group of bladder cancer in community health centers, and screening of common people and high risk practitioners of bladder cancer in physical examination center.
Owner:SHANGHAI INST OF ONCOLOGY
Marker of prostate cancer
InactiveUS20100303795A1Increasing transcriptionalImprove translationOrganic active ingredientsSugar derivativesDisease outcomeProstate cancer
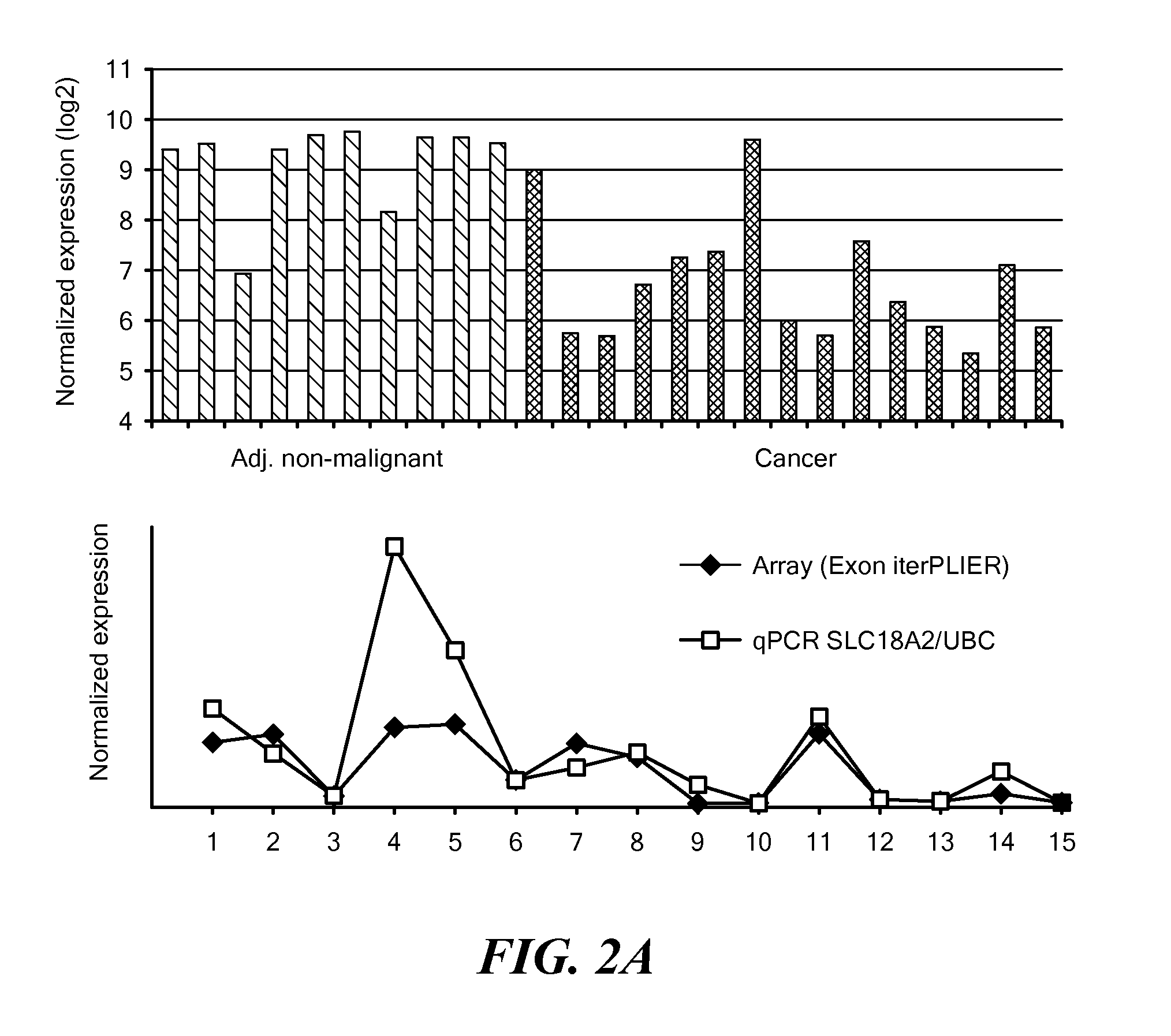
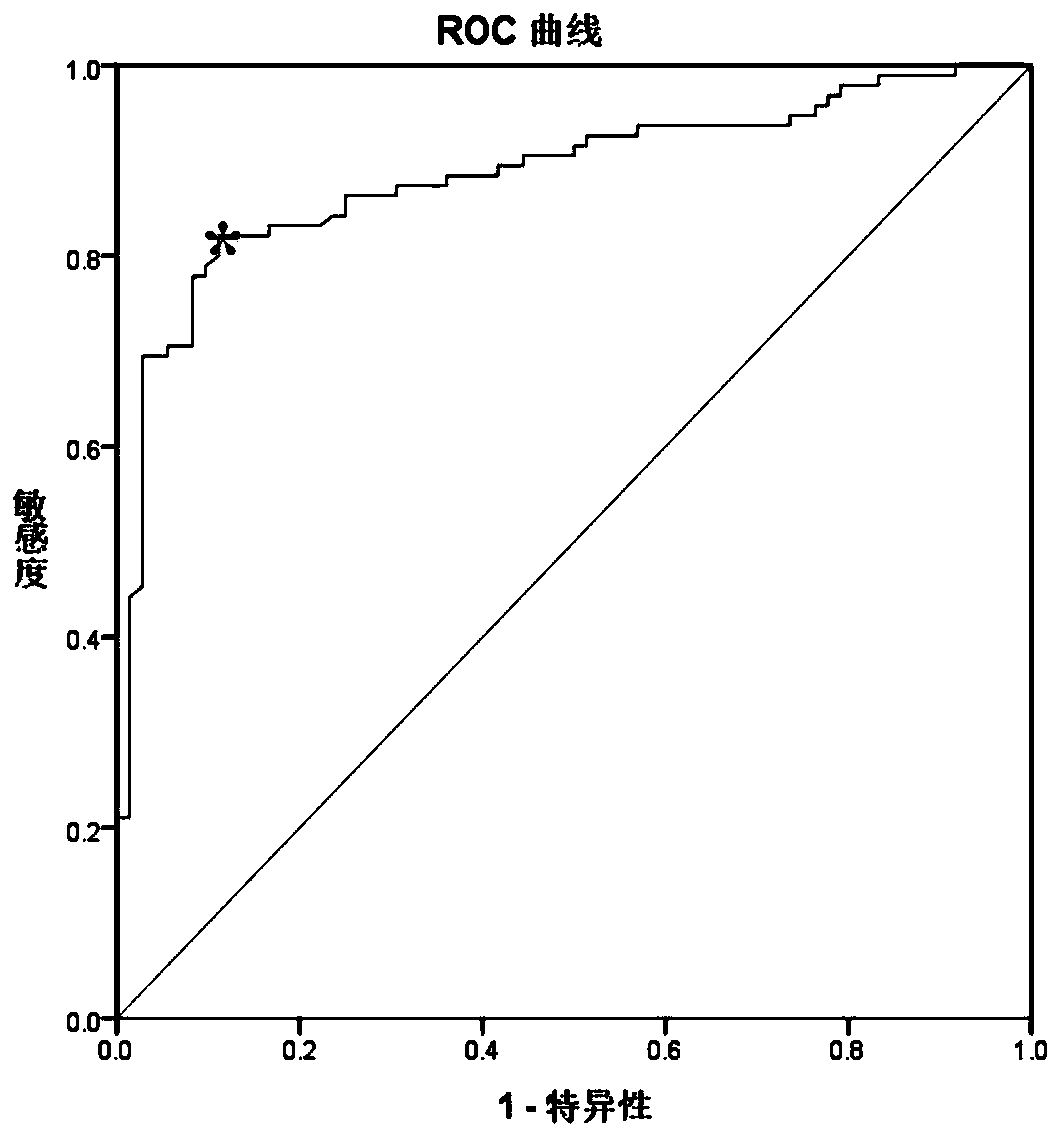
An SLC18A2 gene serves as a marker of prostate cancer. Methods are provided for diagnosing prostate cancer, predicting or prognosticating the disease outcome, predicting recurrence following surgery, and monitoring disease progression in an individual having prostate cancer. The methods relate to determining the methylation state of an SLC18A2 gene and / or determining the level of transcription or translation of the gene in a sample from the individual. Methods of treating prostate cancer are also provided. The invention also pertains to compositions and kits for use in the methods.
Owner:AARHUS UNIV +1
Methylation gene related to lung cancer and detection kit of gene
PendingCN110317875AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCytosineFhit gene
The invention discloses a methylation gene related to the lung cancer and a detection kit of the gene. The gene comprises HOXA7, HOXA9, SCT and SHOX2, and methylation cytosine loci of the gene are calibrated in a sequence 1, a sequence 2, a sequence 3 and a sequence 4 in sequence. The detection kit comprises a reagent for detecting the HOXA7 gene, a reagent for detecting the HOXA9 gene, a reagentfor detecting the SCT and a reagent for detecting the SHOX2 gene. The reagent for detecting the HOXA7 gene comprises primers shown in sequences with the numbers 1-1 and 1-2 and a probe with a sequenceshown in the number 1-3; the reagent for detecting the HOXA9 gene comprises primers shown in sequences with the numbers 2-1 and 2-2 and probes shown in sequences with the number 2-3; the reagent fordetecting the SCT comprises primers shown in sequences with the numbers 3-1 and 3-2, a probe shown in a sequence with the number 3-3, primers shown in sequences with the numbers 3-4 and 3-5 and a probe shown in a sequence with the number 3-6; the reagent for detecting the SHOX2 gene comprises primers shown in sequences with the numbers 4-1 and 4-2 and a probe shown in a sequence with the number 4-3.
Owner:苏州呼呼健康科技有限公司
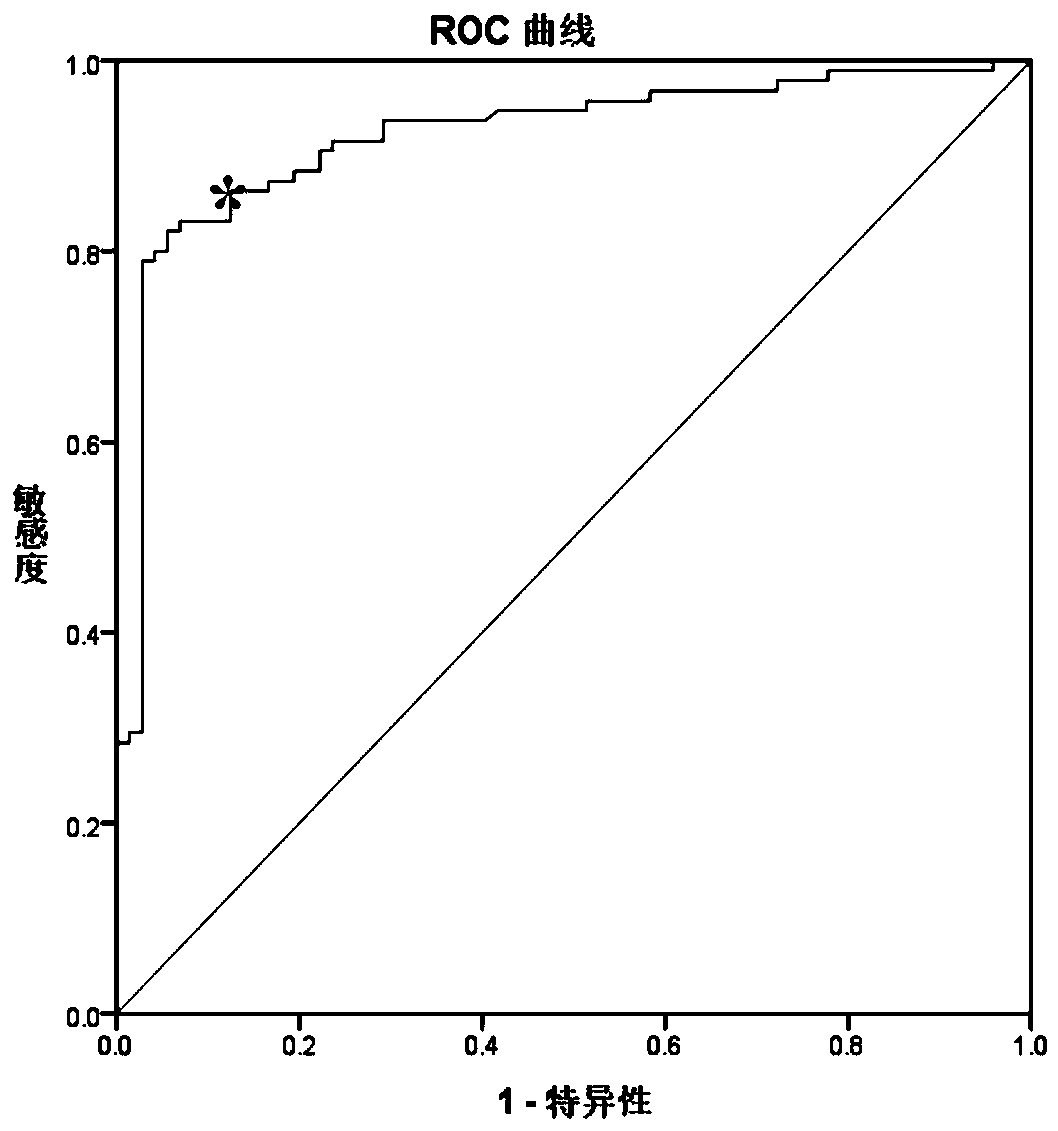
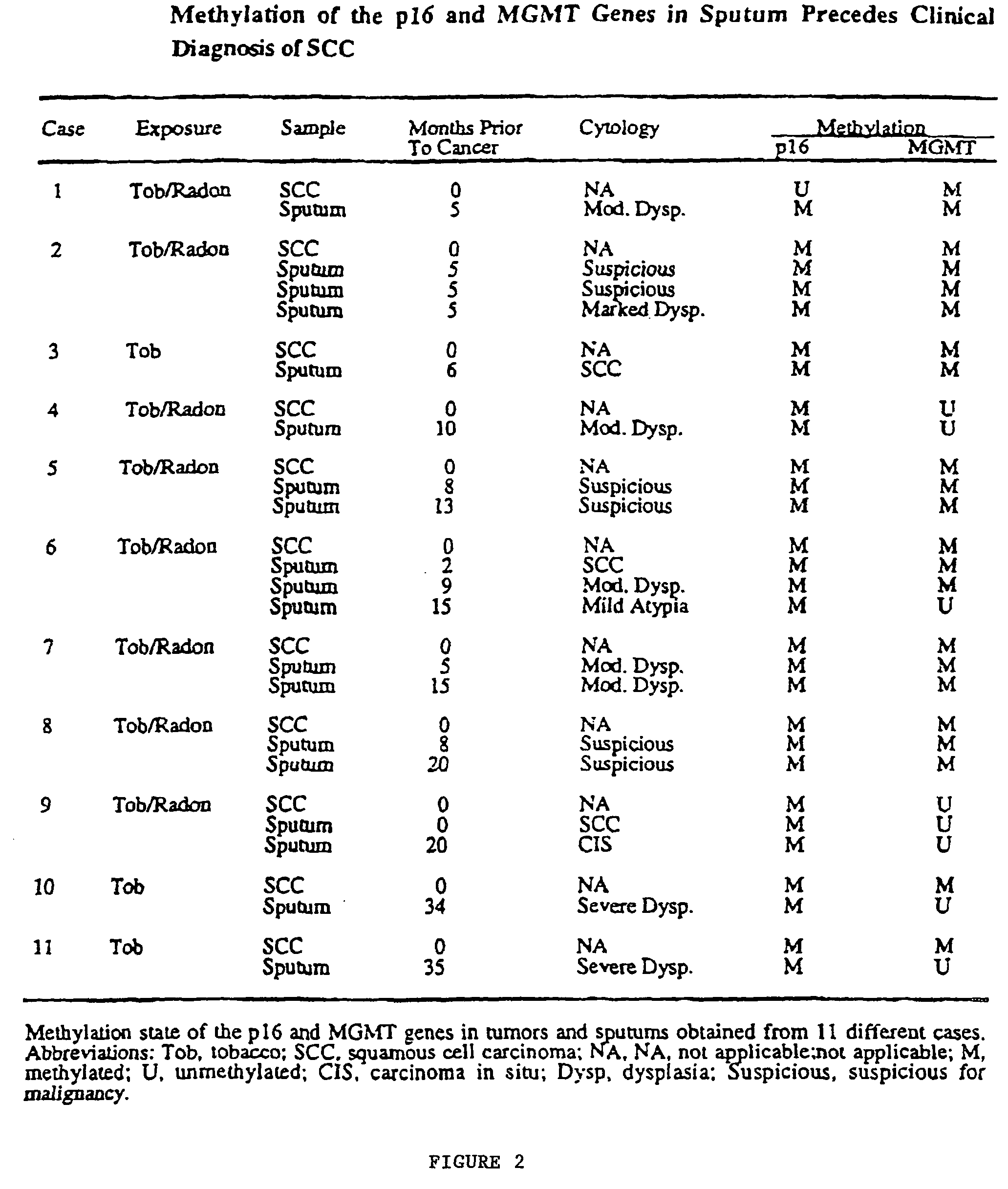
Nested methylation-specific polymerase chain reaction cancer detection method
A molecular marker-based method for monitoring and detecting cancer in humans. Aberrant methylation of gene promoters is a marker for cancer risk in humans. A two-stage, or “nested” polymerase chain reaction method is disclosed for detecting methylated DNA sequences at sufficiently high levels of sensitivity to permit cancer screening in biological fluid samples, such as sputum, obtained non-invasively. The method is for detecting the aberrant methylation of the p16 gene, O 6-methylguanine-DNA methyltransferase gene, Death-associated protein kinase gene, RAS-associated family 1 gene, or other gene promoters. The method offers a potentially powerful approach to population-based screening for the detection of lung and other cancers.
Owner:LOVELACE RESPIRATORY RES INST
DNA methylation markers based on epigenetic stem cell signatures in cancer
ActiveUS20100172880A1Guaranteed maximum utilizationPoor outcomeBiocideMicrobiological testing/measurementDNA methylationNon targeted
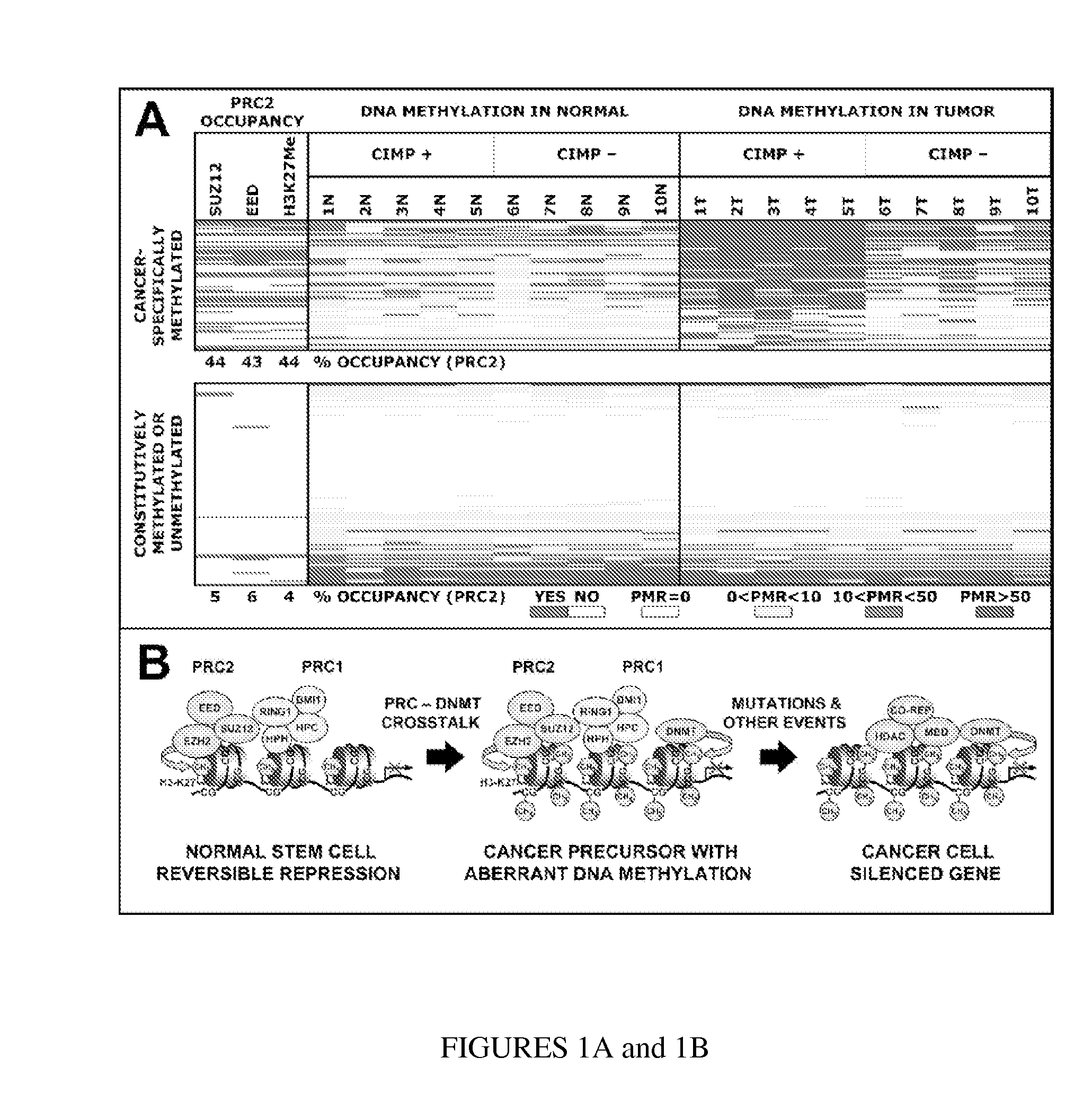
In particular aspects, stem-cell polycomb group (PcG) targets are more likely to have cancer-specific promoter DNA methylation than non-targets, indicating a stem-cell origin of cancer, where reversible gene repression is replaced by permanent silencing, locking the cell into a perpetual state of self-renewal and predisposition to subsequent malignant transformation. Exemplary aspects provide methods for identifying preferred DNA methylation markers for a cellular proliferative disorder and / or cancer and markers for developmental lineages and / or stages, based on identifying PcG protein or PcG repressive complex genomic target loci within a precursor cell (e.g., stem or progenitor cell) population, and determining, in cells of the proliferative disorder and / or cancer or cell of the particular developmental lineages and / or stages, a characteristic methylation status of the PcG target loci. Additional aspects provide methods for validating and / or monitoring a precursor cell (e.g., stem cell) population. Diagnostic and prognostic methods for ovarian and breast cancer are provided.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Methylation Biomarkers for Diagnosis of Prostate Cancer
InactiveUS20140094380A1Increase of methylationMicrobiological testing/measurementLibrary screeningOncologyBiomarker (petroleum)
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
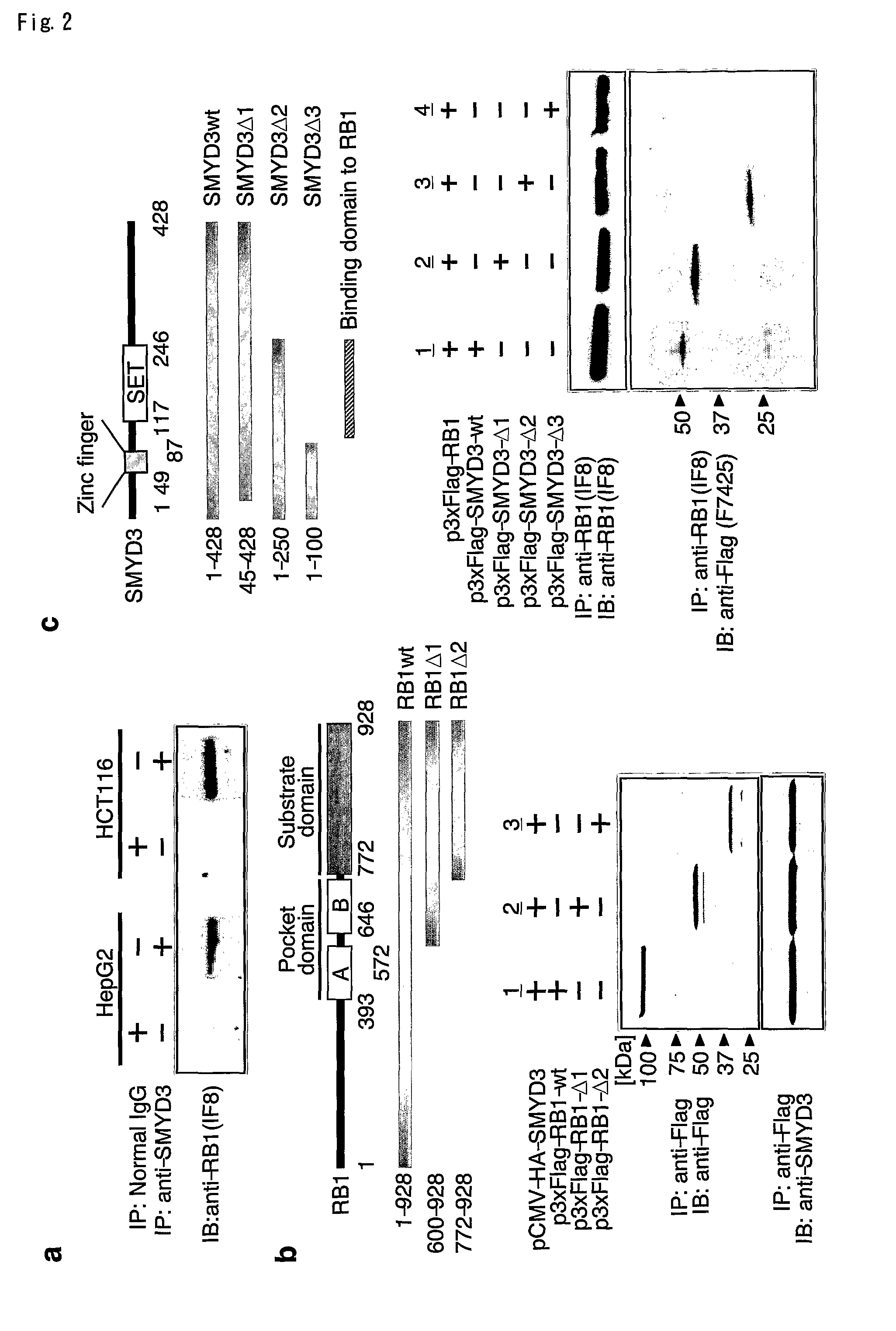
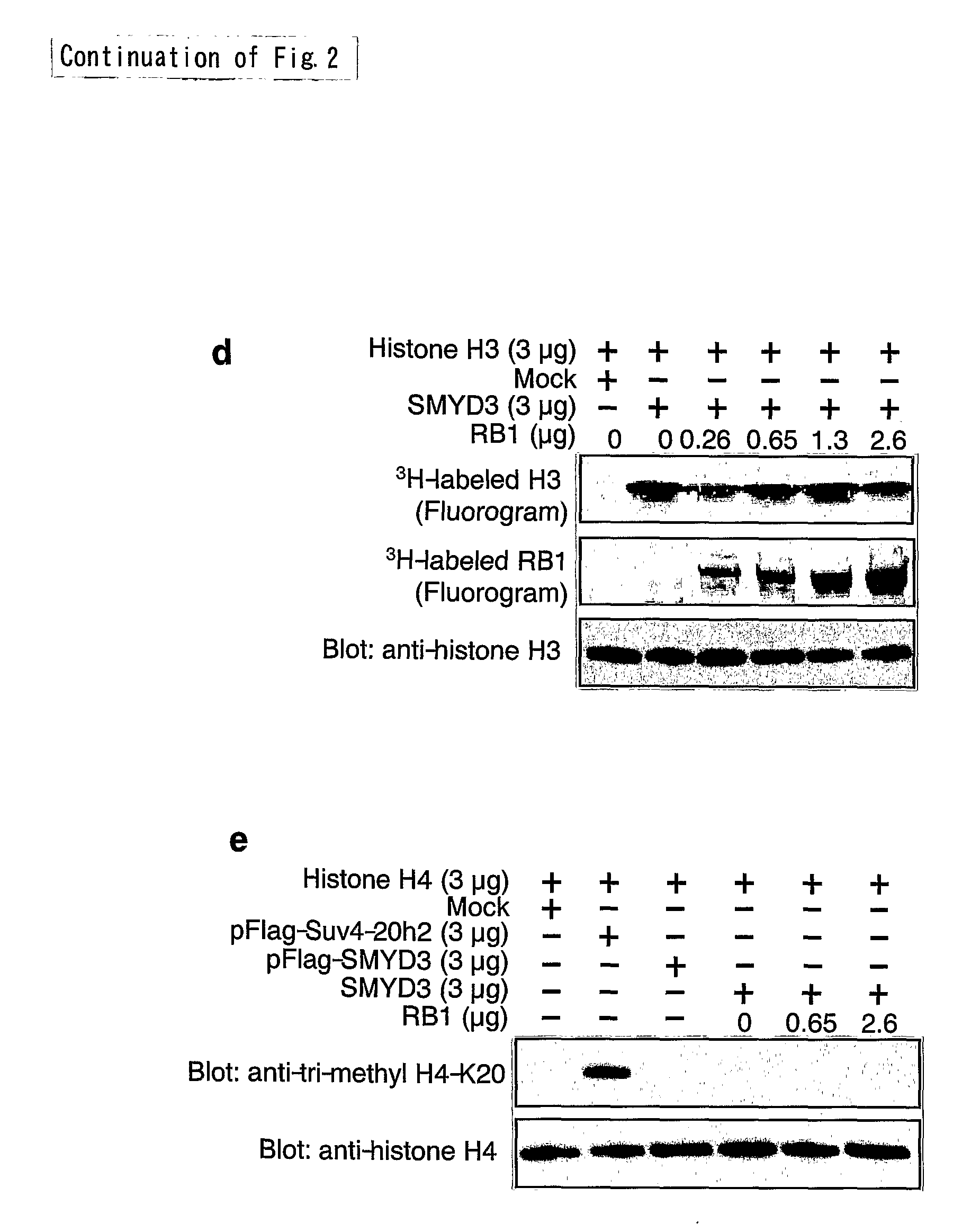
Methods of modulating smyd3 for treatment of cancer
InactiveUS20090191181A1Lower methylation levelsRelieve symptomsCompound screeningApoptosis detectionBladder cancerHepatocellular carcinoma
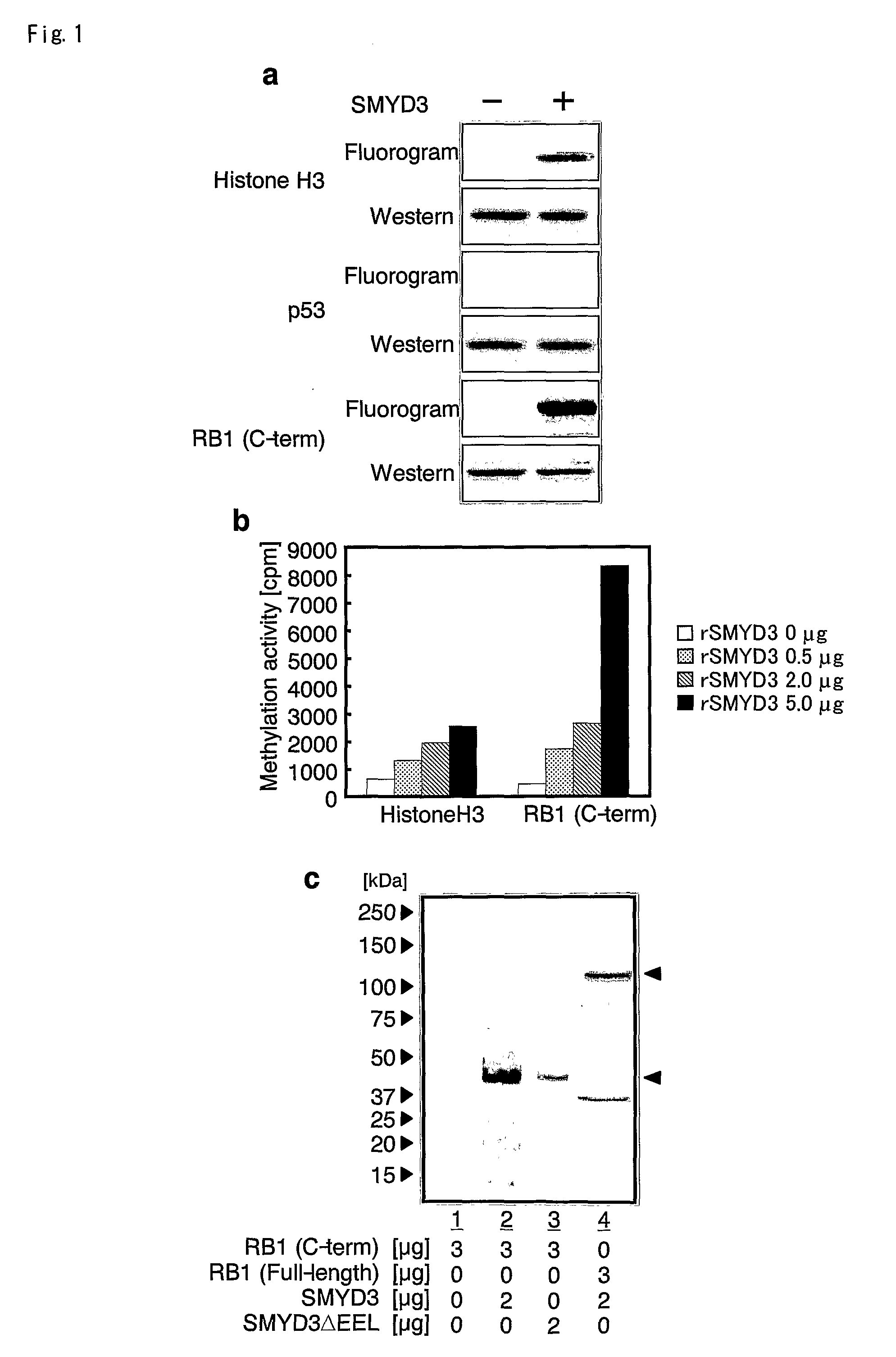
The present invention features a method for determining the methyltransferase activity of a polypeptide and screening for modulators of methyltransferase activity, more particularly for modulators of the methylation of retinoblastoma by SMYD3. The invention further provides a method or pharmaceutical composition for prevention or treating of colorectal cancer, hepatocellular carcinoma, bladder cancer and / or breast cancer using a modulator so identified. N-terminal truncated forms of SMYD3 (alias ZNFN3A1) have higher methylating activity. Lys 824 is a preferred methylation site on the RB1 protein for SMYD3.
Owner:ONCOTHERAPY SCI INC
Method for detecting the methylation of colon-cancer-specific methylation marker genes for colon cancer diagnosis
ActiveCN102686744AFast and Accurate DiagnosisEffective diagnosisMicrobiological testing/measurementDNA/RNA fragmentationCancers diagnosisColon cancer cell
The present invention relates to a method for detecting the methylation of colon-cancer-specific methylation marker genes for colon cancer diagnosis, and more particularly, to a method which involves detecting the methylation of colon-cancer-specific methylation marker genes which are specifically methylated in colon cancer cells, and providing information for colon cancer diagnosis. Using the methylation detection method, and a composition, a kit and a nucleic acid chip for diagnosis according to the present invention, colon cancer can be diagnosed in an early transformation stage to enable the early diagnosis of colon cancer. The method of the present invention enables the diagnosis of colon cancer to be performed in a more accurate and quick manner as compared to conventional methods, and therefore can be effectively used.
Owner:GENOMICTREE
Early Detection and Prognosis of Colon Cancers
InactiveUS20080221056A1Organic active ingredientsGenetic material ingredientsGenes mutationBiomarker (petroleum)
We have developed a transcriptome-wide approach to identify genes affected by promoter CpG island hypermethylation and transcriptional silencing in colorectal cancer (CRC). By screening cell lines and validating tumor specific hypermethylation in a panel of primary human CRC samples, we estimate that nearly 5% of all known genes may be promoter methylated in an individual tumor. When directly compared to gene mutations, we find a much larger number of genes hypermethylated in individual tumors, and much higher frequency of hypermethylation within individual genes harboring either genetic or epigenetic changes. Thus, to enumerate the full spectrum of alterations in the human cancer genome, and facilitate the most efficacious grouping of tumors to identify cancer biomarkers and tailor therapeutic approaches, both genetic and epigenetic screens should be undertaken. The genes we identified can be used inter alia diagnostically to detect cancer, pre-cancer, and likelihood of developing cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
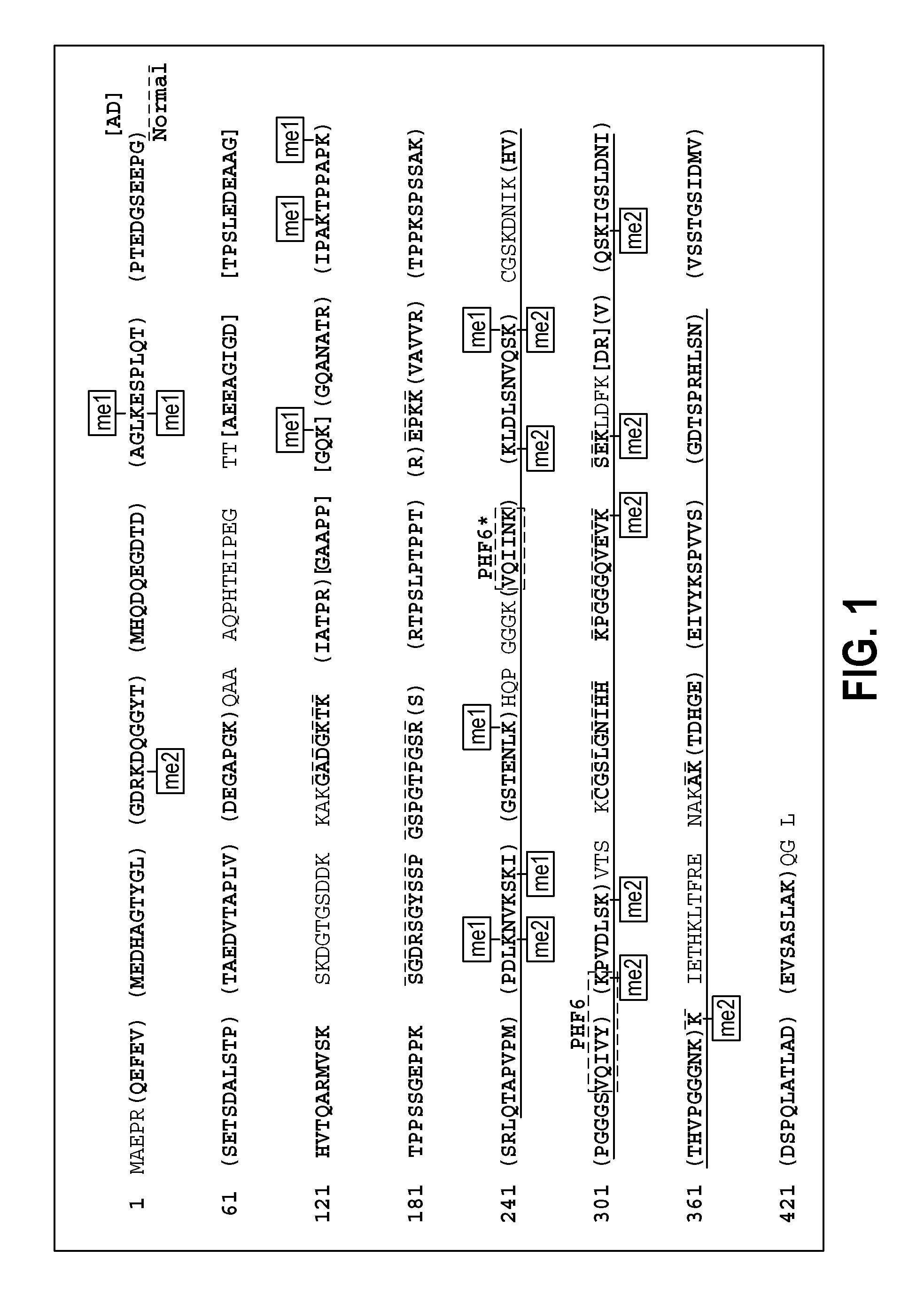
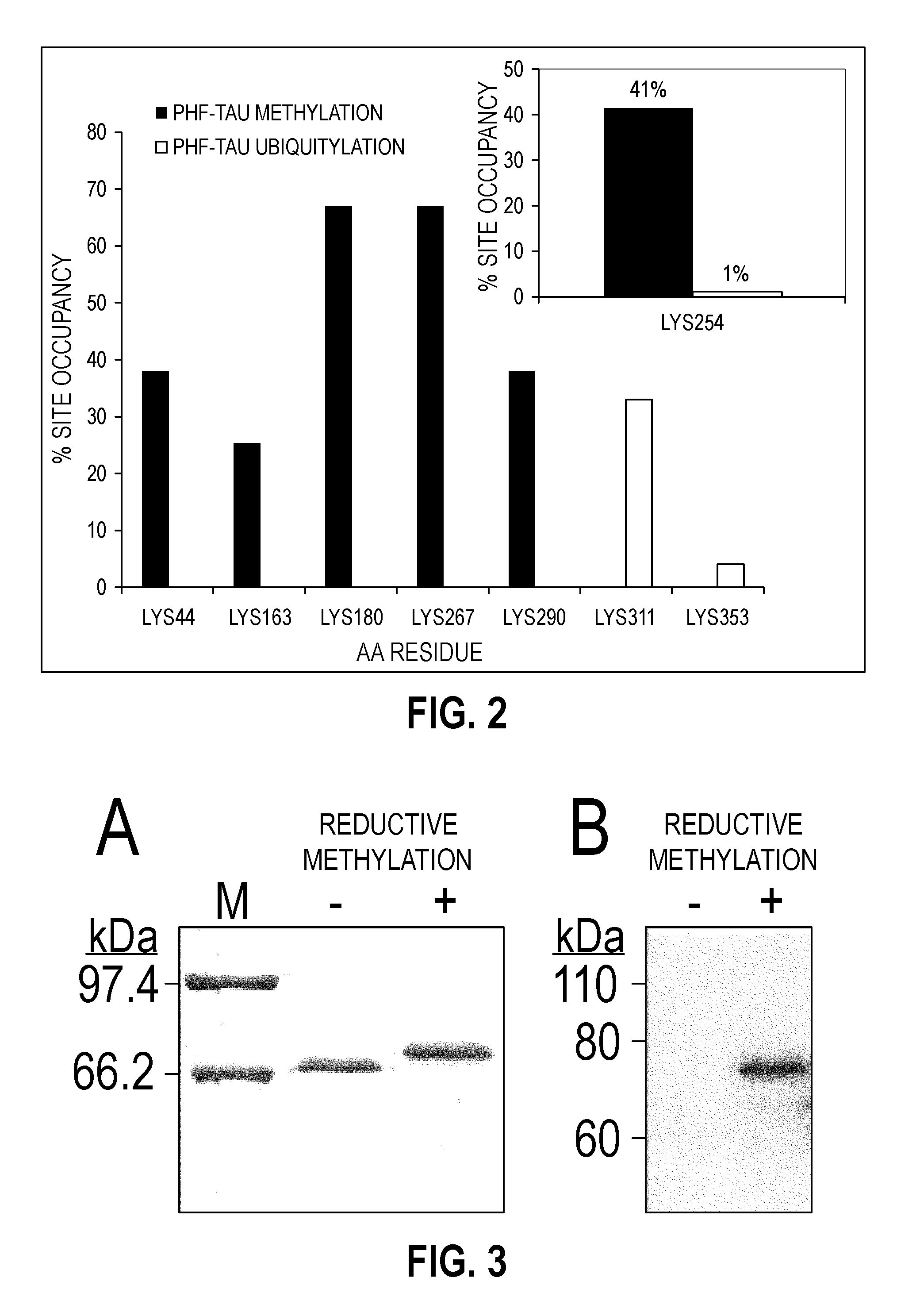
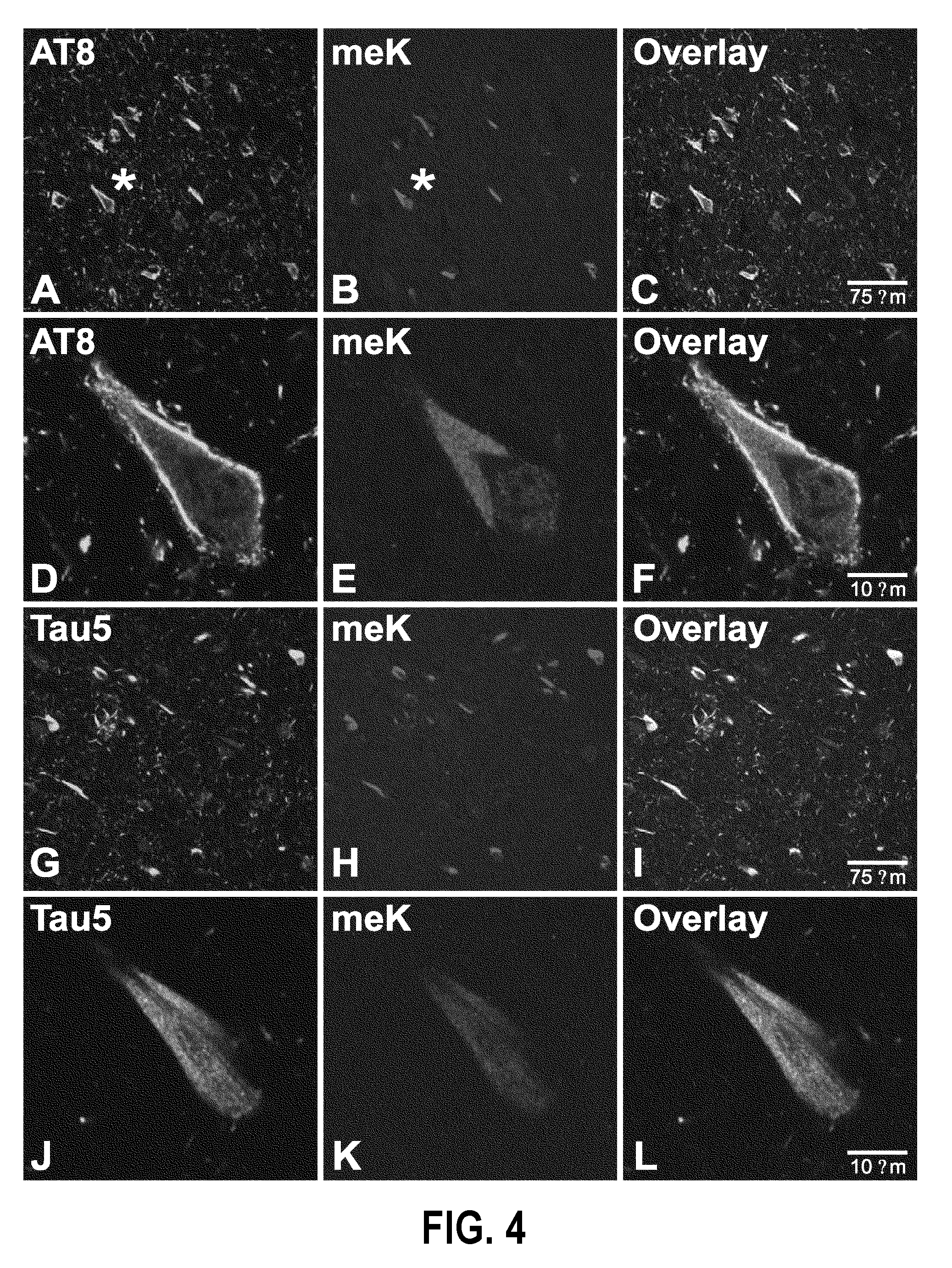
Methylated Peptides Derived from Tau Protein and Their Antibodies for Diagnosis and Therapy of Alzheimer's Disease
ActiveUS20140294839A1Low extensionImprove understandingNervous disorderPeptide/protein ingredientsAntigenPost translational
In sporadic Alzheimer's disease, neurofibrillary lesion formation is preceded by extensive post-translational modification of the microtubule associated protein tau. Immunoassays have been developed recently that detect tau in biological specimens, thus providing a means for pre-mortem diagnosis of Alzheimer's disease, which has remained elusive. These assays have been improved by the analysis of relevant post-translational modifications, such as phosphorylation, however opportunity for improvement remains. The present invention addresses this issue by disclosing synthetic methylated peptides derived from the tau protein of paired helical filaments and non-diseased control brain. Alzheimer's disease specificity is provided by the presence or absence of methyl moieties on lysine residues and differences between mono-, di-, and tri-methylation. The methylated peptide is useful as an antigen and a binding partner for identifying compounds that interact with the peptide and the methylated tau protein, including antibodies that can distinguish non-diseased brain from that affected by Alzheimer's disease. The resulting antibodies are useful diagnostically and therapeutically. The compounds that specifically bind to methylated tau proteins are useful for eliminating abnormally methylated tau.
Owner:UNIV OF MARYLAND BALTIMORE +1
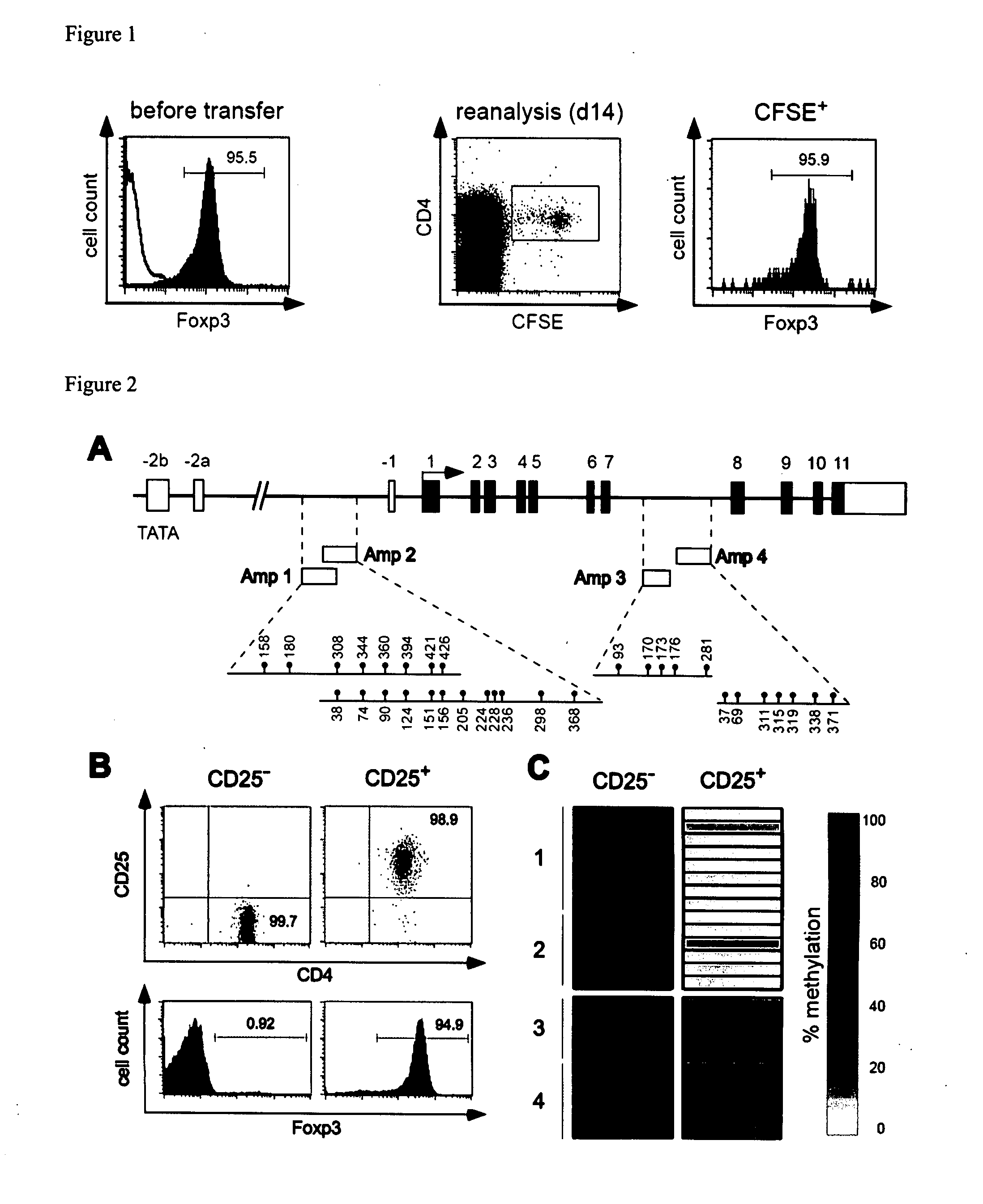
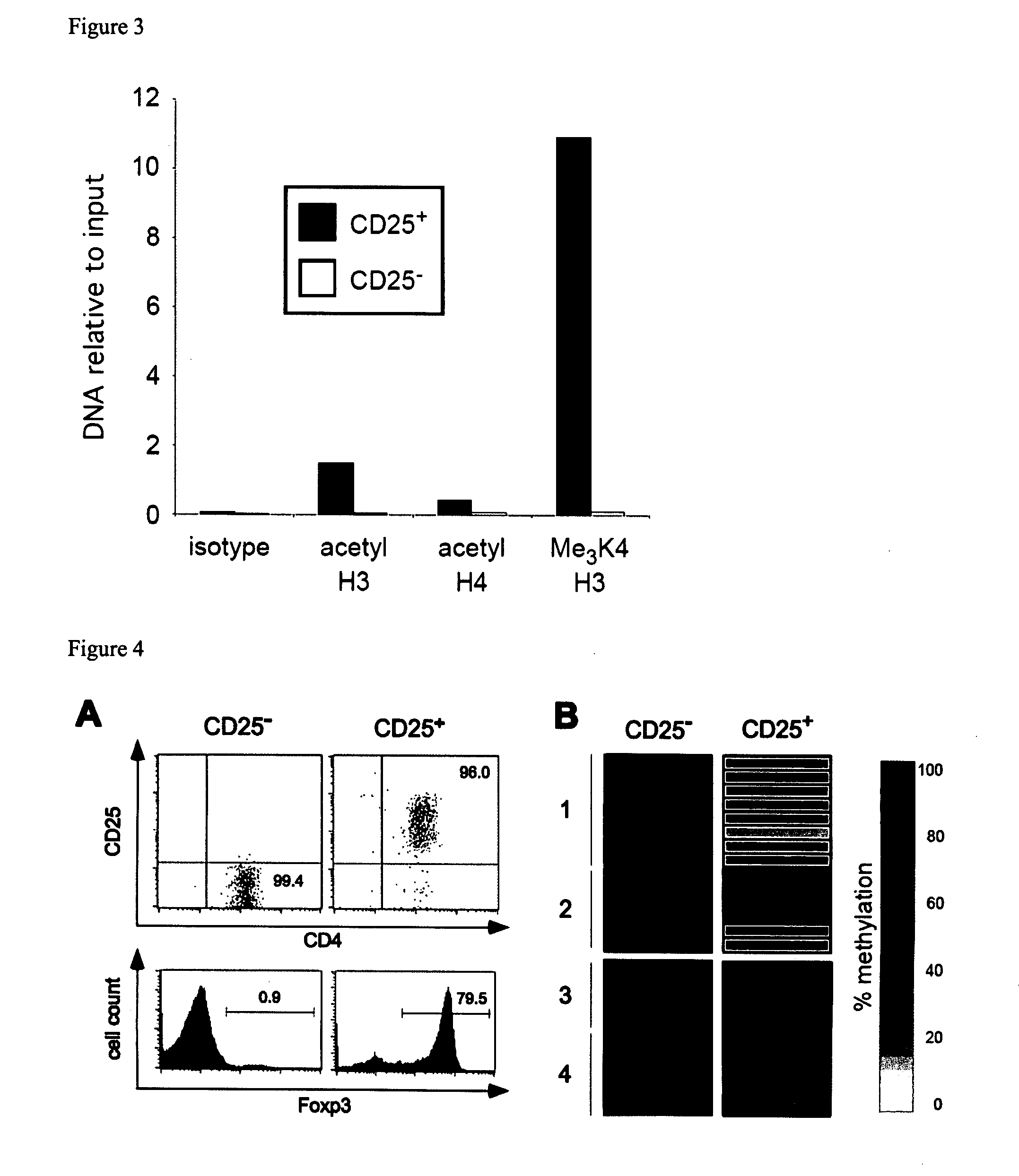
Epigenetic modification of the loci for CAMTA1 and/or FOXP3 as a marker for cancer treatment
PendingUS20070243161A1Short overall survivalConvenient treatmentOrganic active ingredientsPeptide/protein ingredientsDiseaseRegulatory T cell
The present invention relates to a method, in particular an in vitro method, for pan-cancer diagnostics, comprising identifying the amount and / or proportion of stable regulatory T cells in a patient suspected of having cancer through analyzing the methylation status of at least one CpG position in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, wherein an increased amount and / or proportion of stable regulatory T cells in said patient is indicative for an unspecific cancerous disease. In a second aspect thereof, the present invention relates to a method for diagnosing the survival of a cancer patient, comprising identifying the amount and / or proportion of stable regulatory T cells in said cancer patient through analyzing the methylation status of at least one CpG position in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, wherein a demethylation in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, is indicative of a stable regulatory T cell, and wherein an increased amount and / or proportion of stable regulatory T cells in said cancer patient is indicative for a shorter survival for said cancer patient. Furthermore, the present invention relates to an improved treatment of cancers based on the inventive methods, and a kit for performing the above methods as well as respective uses.
Owner:PRECISION FOR MEDICINE GMBH
Use of ID4 for diagnosis and treatment of cancer
ActiveUS20070020646A1Lower methylation levelsImprove expression levelSugar derivativesMicrobiological testing/measurementBreast cancer metastasisLymphatic Spread
Methods for diagnosis and treatment of cancer using ID4 are disclosed. Specifically, epigenetic inactivation of ID4 in colorectal carcinomas and breast correlates with poor differentiation and unfavorable prognosis. Further, aberrant hypermethylation of ID4 gene promoter region increases risk of metastasis in colorectal and breast cancer.
Owner:JOHN WAYNE CANCER INST
Methylation markers for diagnosis and treatment of ovarian cancer
InactiveUS7507536B2Reducing and inhibiting neoplastic growthRestoring expression of the polypeptide in the cellMicrobiological testing/measurementMaterial analysisSilencing genePolynucleotide
Owner:MDXHEALTH
Method for detecting gastric polyp and gastric cancer marker gene of gastric polyp and gastric cancer-specific methylation
The present invention relates to the novel use of syndecan-2 (SDC2; NM_002998) gene as a gastric polyp- and gastric cancer-specific methylation biomarker, and more particularly, to the use of the syndecan-2 gene as a biomarker that enables gastric polyp and gastric cancer to be diagnosed in an early stage by measuring the methylation level thereof. The present invention has an effect in that the methylation of the CpG island of the gastric polyp- and gastric cancer-specific marker gene can be detected to thereby provide information for diagnosing gastric cancer. The use of the methylation detection method according to the present invention or the diagnostic composition, kit or nucleic acid chip according to the present invention makes it possible to diagnose gastric cancer at an early transformation stage, thus enabling the early diagnosis of gastric cancer. In addition, the method of the present invention enables gastric cancer to be effectively diagnosed in an accurate and rapid manner compared to conventional methods.
Owner:GENOMICTREE
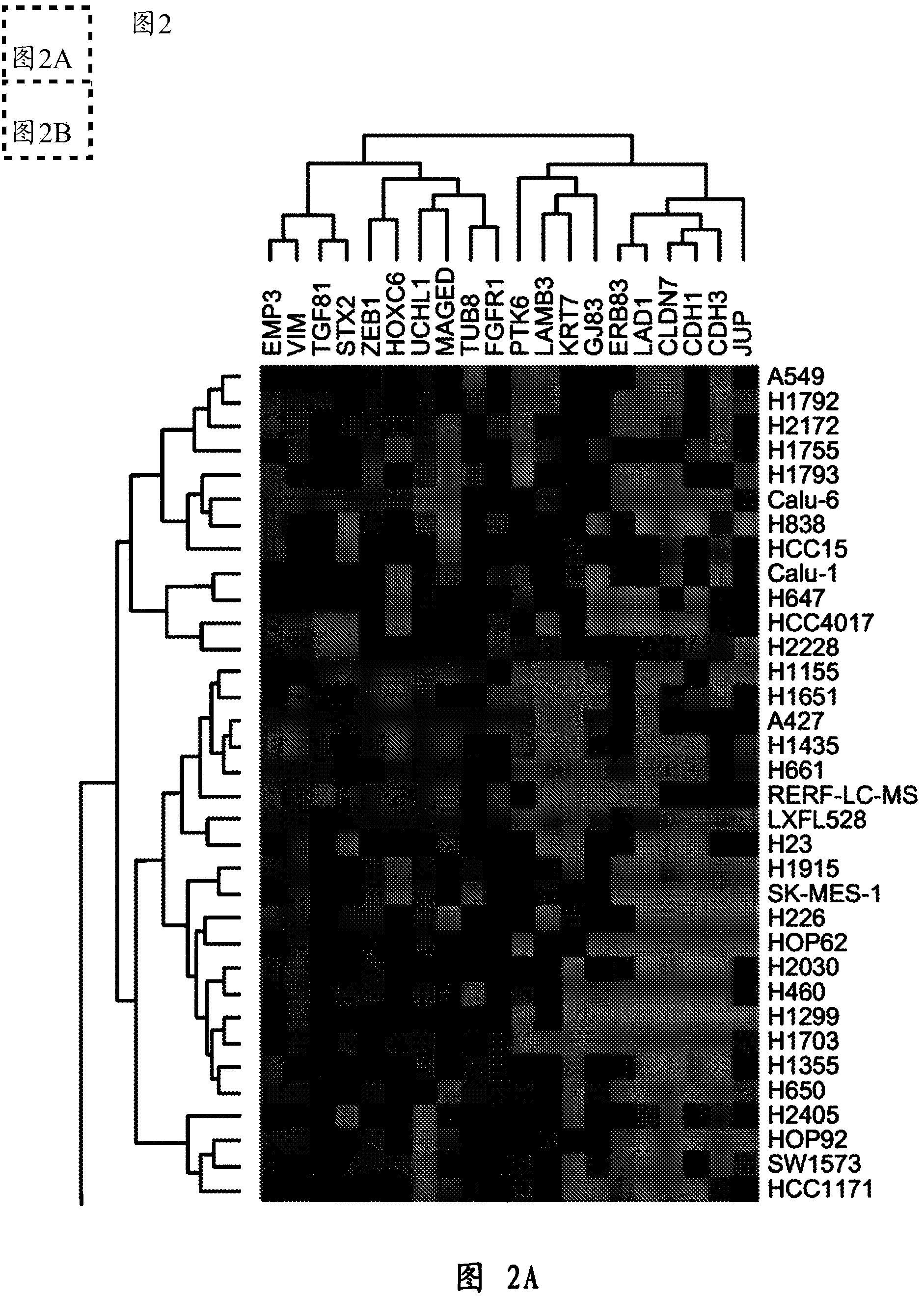
Diagnostic methylation markers of epithelial or mesenchymal phenotype and response to EGFR kinase inhibitor in tumours or tumour cells
Owner:GENENTECH INC
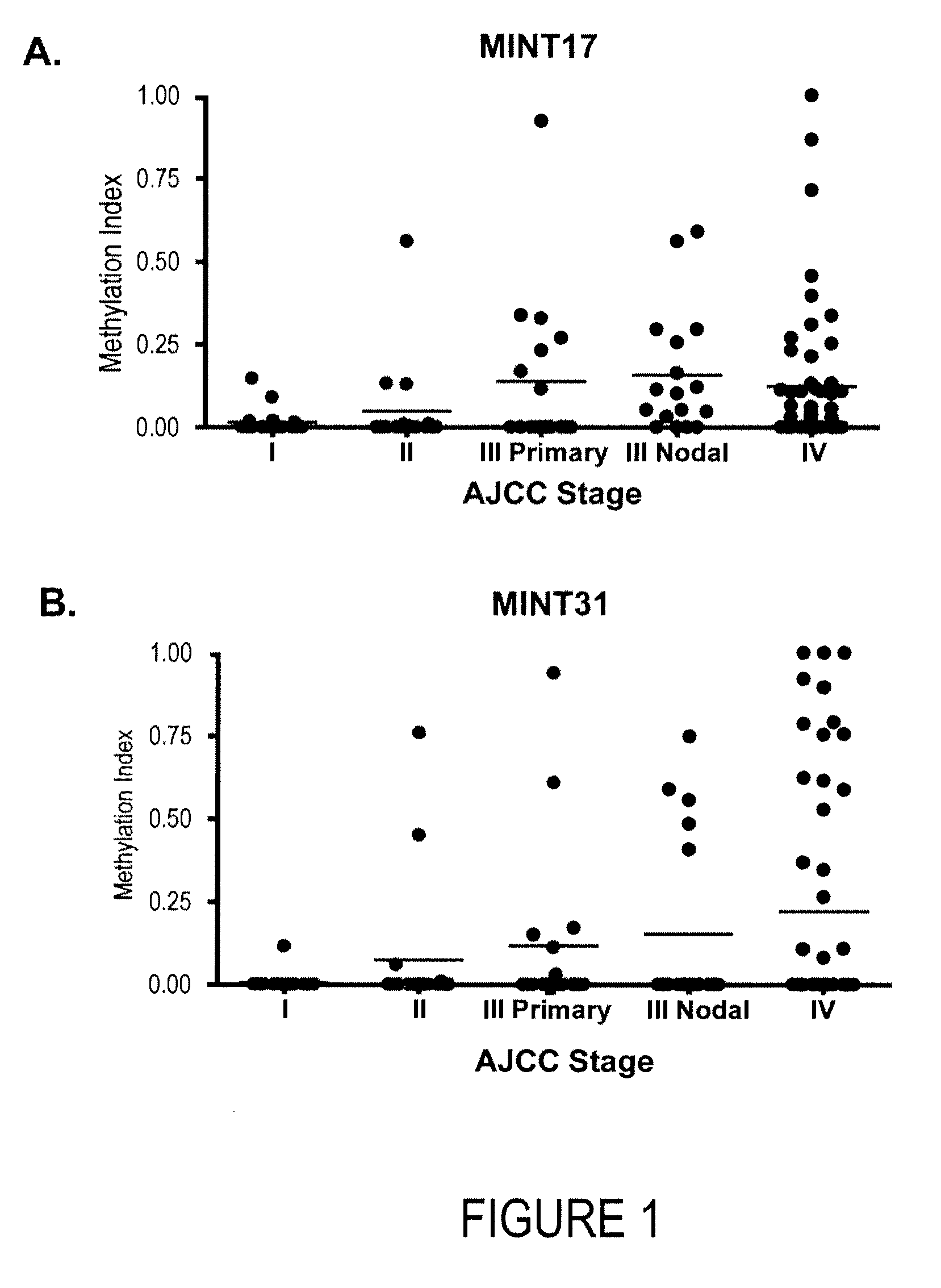
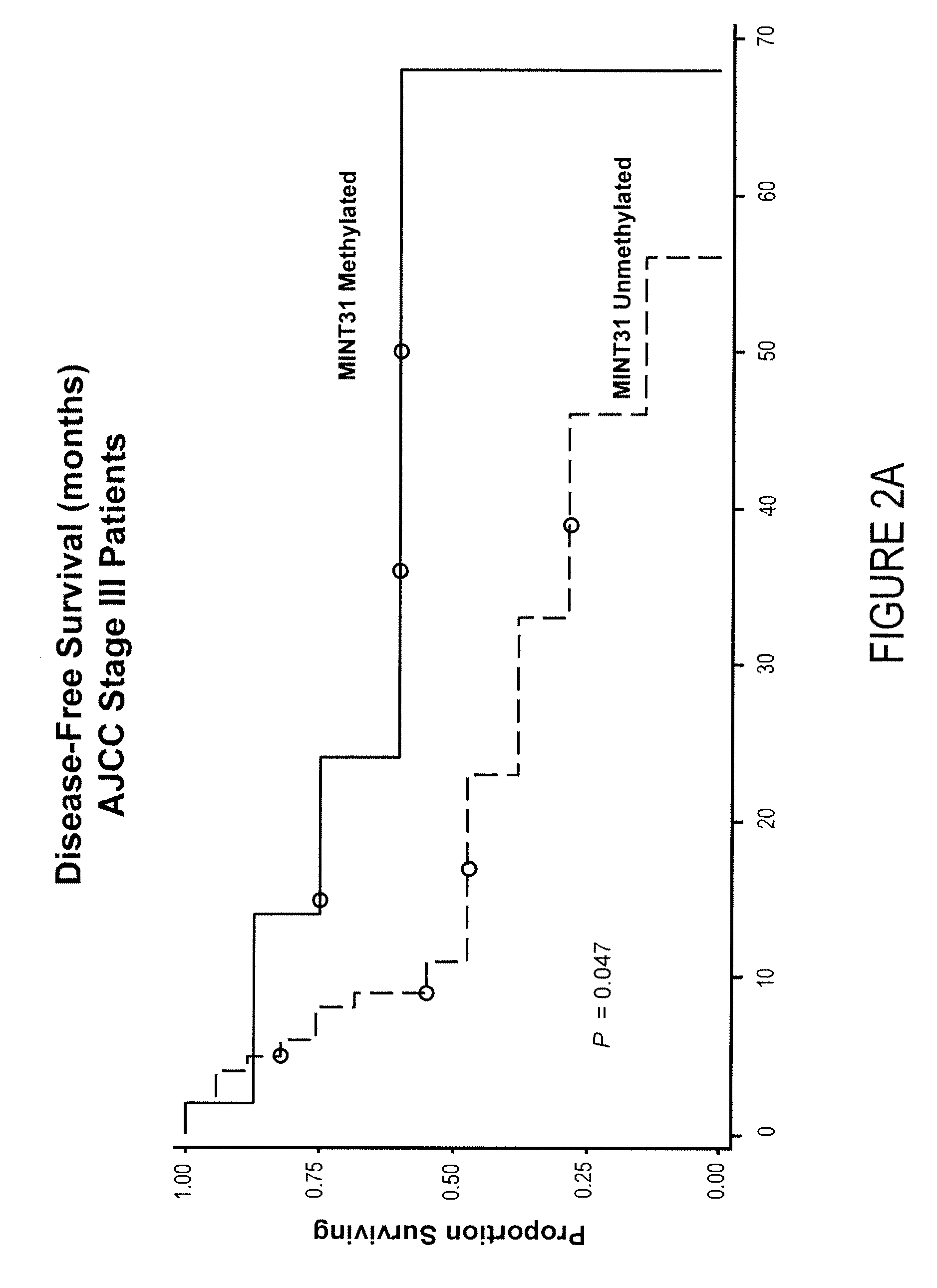
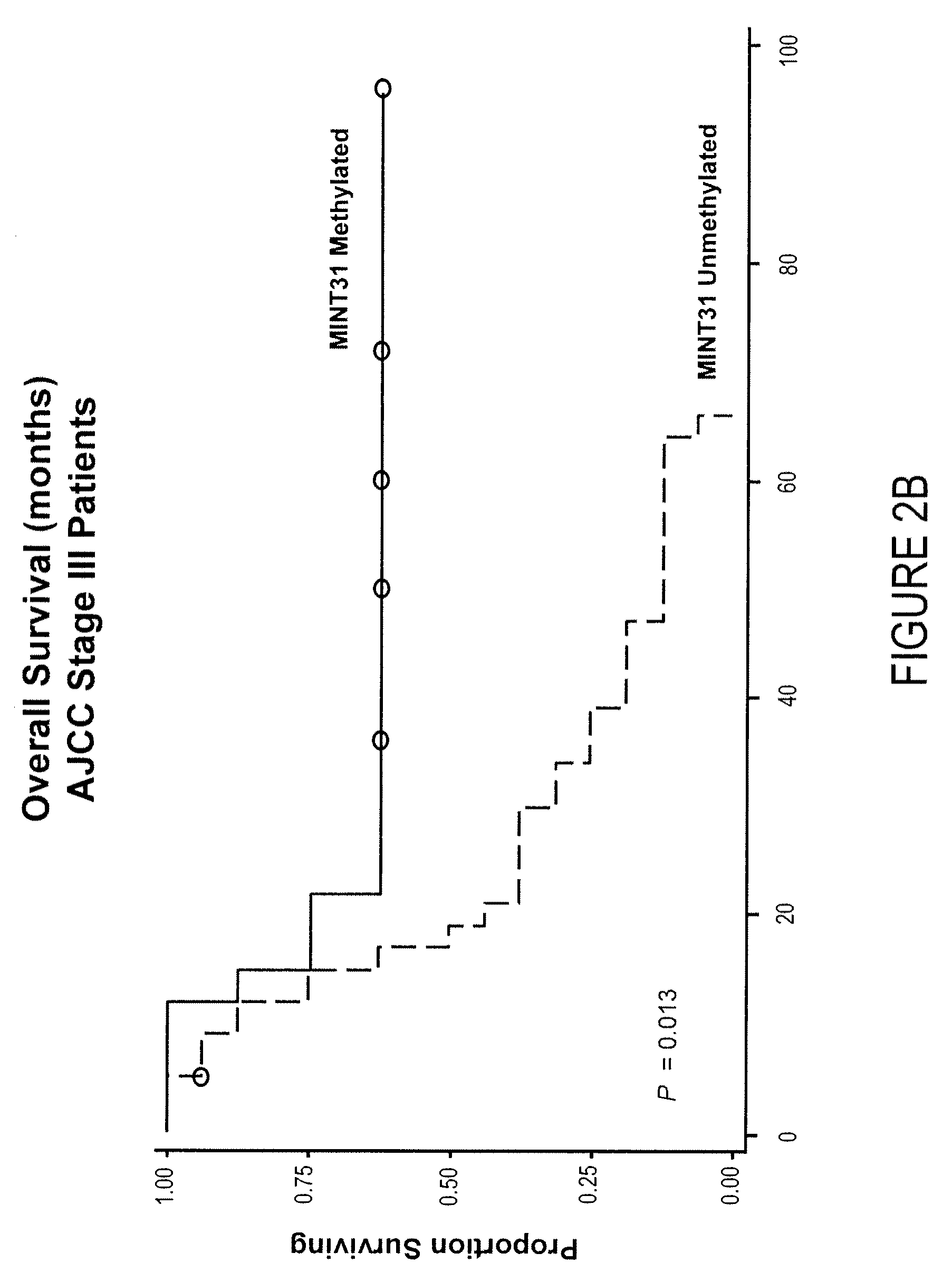
Use of methylation status of mint loci and tumor-related genes as a marker for melanoma and breast cancer
InactiveUS20090220980A1Less likelihood of overall survivalHigh level of methylationMicrobiological testing/measurementMelanomaOncology
The invention relates to a method of detecting melanoma or breast cancer using DNA methylation in MINT17, MINT31, or the promoter region of WIF1, TFPI2, RASSF1A, SOCS1, GATA4, or RARβ2 as a biomarker. Also disclosed are methods of using the biomarker for determining the cancer status and predicting the outcome of the cancer.
Owner:JOHN WAYNE CANCER INST
Application of specific methylation sites as breast cancer molecular subtype diagnosing markers
InactiveCN108676879AMicrobiological testing/measurementDNA/RNA fragmentationDiseaseBreast cancer subtype
The invention discloses application of specific methylation sites as breast cancer molecular subtype diagnosing markers. The invention establishes a method and a standard for screening disease-relatedchromosomal methylation sites, and screens 40 methylation sites related to breast cancer diagnosis by using breast cancer patients as cases. The screened specific methylation sites as the breast cancer molecular subtype diagnosing markers can be applied to non-invasive detection of molecular breast cancer subtypes and diagnosis of other diseases. A kit is used for one or more of the following applications: diagnosis of the breast cancer molecular subtypes, assessment of breast cancer risks, assessment of a breast cancer treating effect, screening of breast cancer treating medicines and the like.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION
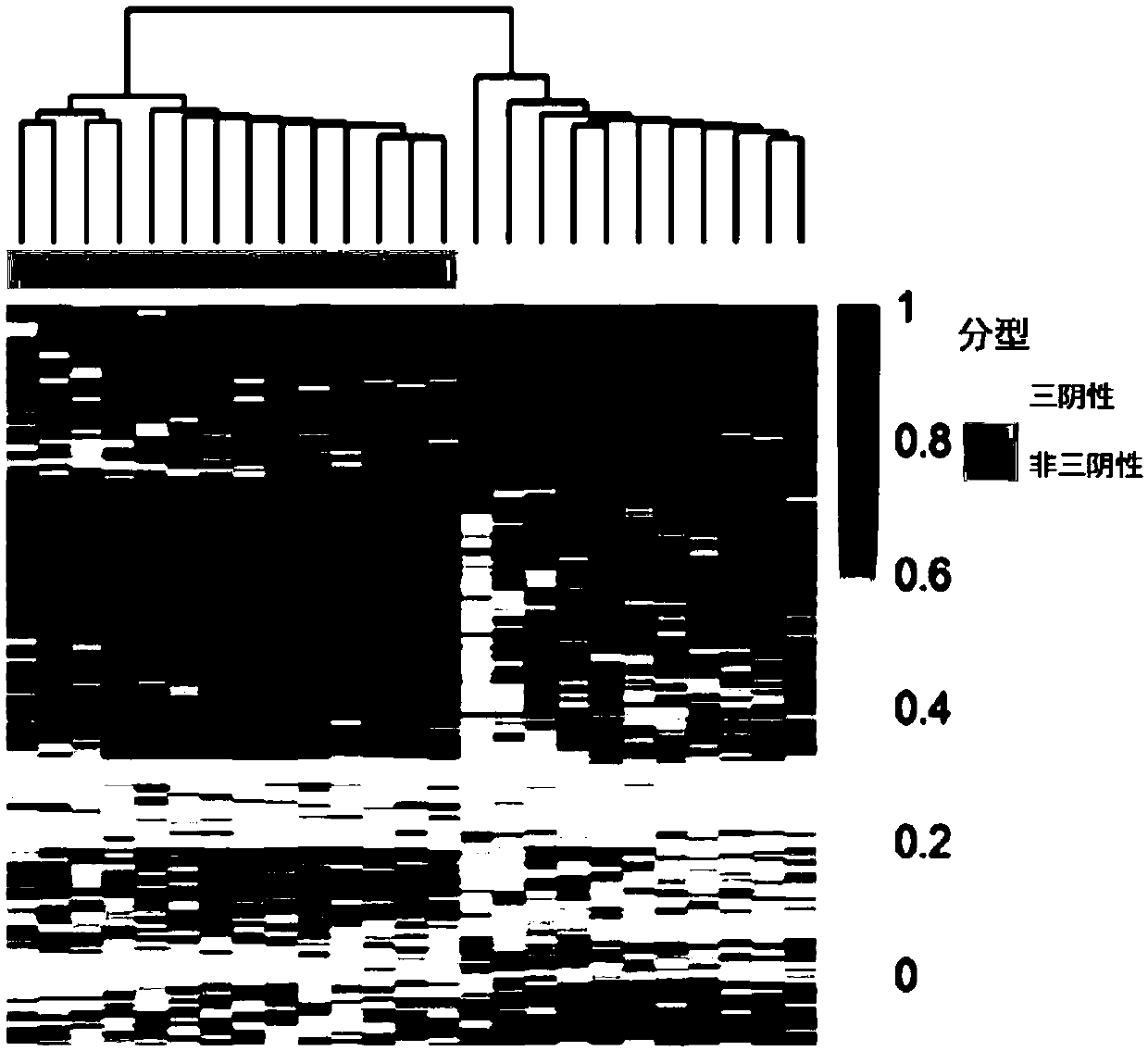
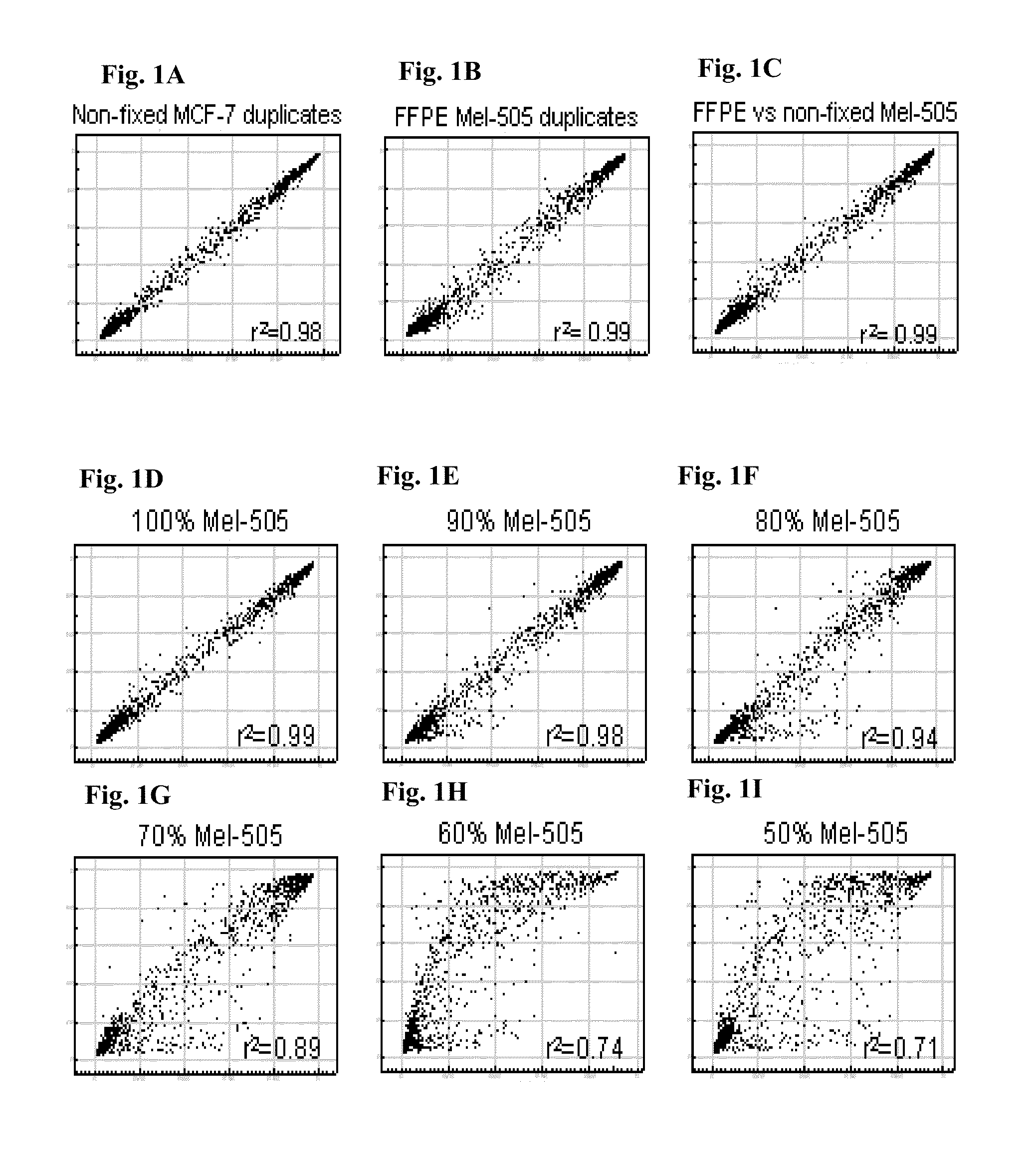
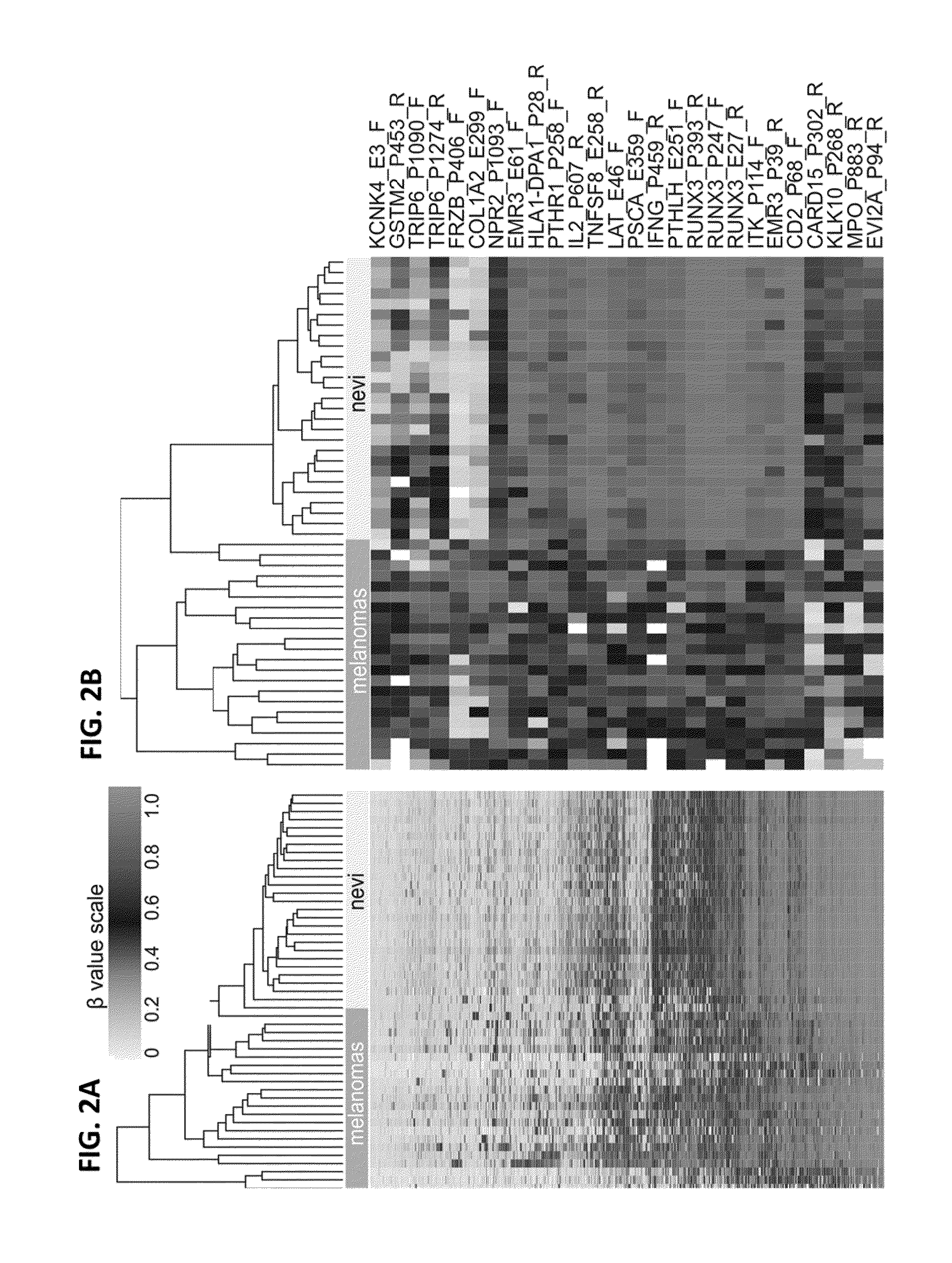
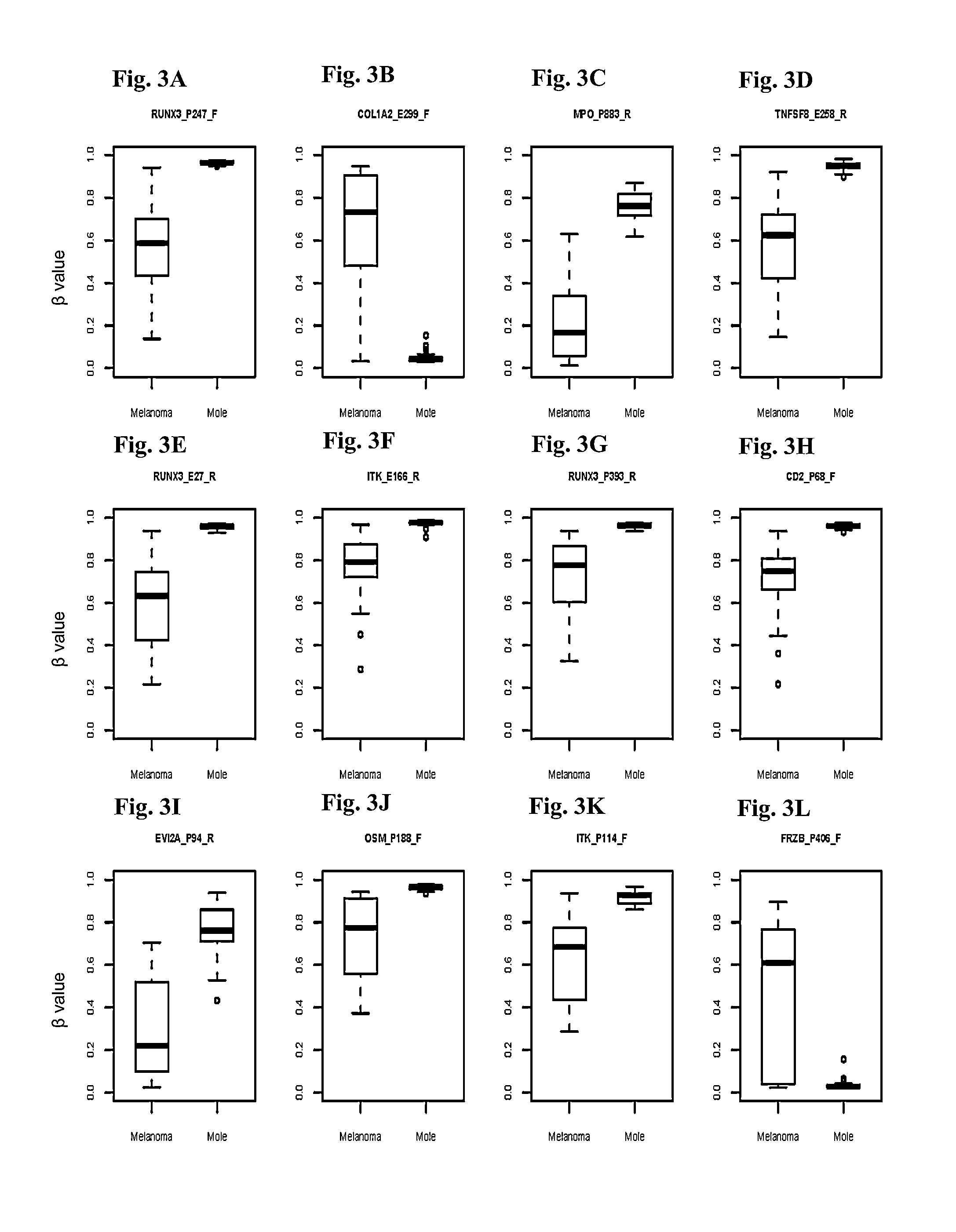
Methods and kits for detecting melanoma
This invention is directed to a method for detecting melanoma in a tissue sample by measuring a level of methylation of one or more regulatory elements differentially methylated in melanoma and benign nevi. The invention provides methods for detecting melanoma, related kits, and methods of screening for compounds to prevent or treat melanoma.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Kits for survival and prognosis of glioblastoma related to SCOS3
The invention provides kits for the survival and the prognosis of glioblastoma related to SCOS3. The kits comprise a kit for extracting DNA from a tumor tissue sample of a patient with primary glioblastoma, an optional instruction manual for the DNA extracting kit, a kit for analyzing the methylation level of gene SOCS3 in the extracted DNA and an optional instruction manual for the kit for analyzing the methylation level of the gene SOCS3. The invention also provides a molecular marker SOCS3 gene for the survival and the prognosis of glioblastoma.
Owner:江涛
Characterizing prostate cancer
InactiveCN101220392AMicrobiological testing/measurementBiological testingRecurrent CancerProstate sample
The invention relates to identify prostate cancer. The invention provides a method for predicting the recurrence or aggressiveness of prostate cancer, comprising a) determining the Gleason score of a prostate sample, and b) determining the methylation status of a Marker in a biological sample for those patients having a Gleason score of 7 or greater; wherein methylation that exceeds a pre-determined value is indicative of an aggressive or recurrent cancer and methylation that does not exceed such pre-determined value is indicative of indolent cancer. The invention also provides a kit used for predicting the course or aggressiveness of the prostate cancer, which comprising nucleic acid amplification test reagent and a synopsis which instructs the patients having the Gleason score of 7 or greater in the use of the reagent.
Owner:VERIDEX LCC
Method for detecting gastric polyp and gastric cancer using marker gene of gastric polyp and gastric cancer-specific methylation
InactiveCN104812899AEffective diagnosisMicrobiological testing/measurementMaterial analysisSyndecan-2Gastric Polyp
The present invention relates to a novel use of the SDC2 (NM_002998, syndecan 2) gene as a biomarker of a gastric polyp and gastric cancer-specific methylation, and more particularly, to a use for diagnosing a gastric polyp and gastric cancer in an early stage by measuring the methylation level using the SDC2 gene as a biomarker. The present invention has the effect of providing a method which can provide information for the diagnosis of gastric cancer by detecting the methylation of a CpG island of a gastric polyp and a gastric cancer-specific marker gene. Also, using the method for detecting methylation and a composition, kit, and nucleic acid chip for diagnosis according to the present invention, gastric cancer can be diagnosed at an initial transformation stage, and thus, early diagnosis can be achieved. Therefore, the present invention is useful as it is possible to diagnose gastric cancer more accurately and rapidly than conventional methods.
Owner:GENOMICTREE
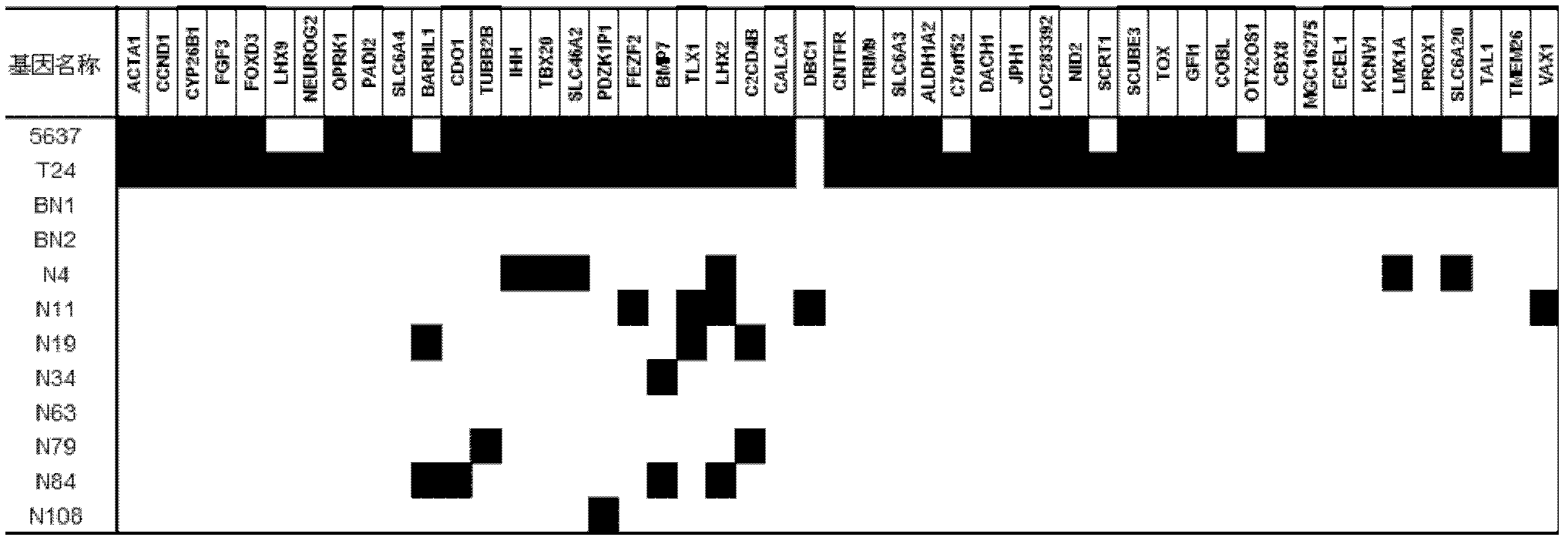
Urine-based method and kit for diagnosing relapse risk of bladder cancer patient
The invention relates to a urine-based method and kit for diagnosing the relapse risk of a bladder cancer patient. The invention discloses two methylation-sensitive genes, namely an LMX1A gene and a VAX1 gene. A urine sample is kept before the operation of a detected bladder cancer patient, and the specific CpG sites of the genes are subjected to high methylation among people who are determined to suffer from a relapse in the follow-up visit process. Thus, the two genes can be used as biological markers for the relapse of bladder cancer. The invention can be used as the basis for designing a kit for prognosing and diagnosing the relapse of bladder cancer, and is applicable to the prognosis detection of bladder cancer in hospital, the postoperative follow-up visit and the postoperative monitoring of the community health center on people subjected to bladder cancer operation.
Owner:SHANGHAI INST OF ONCOLOGY
Aberrantly Methylated Genes as Markers of Breast Malignancy
InactiveUS20090136944A1Sugar derivativesMicrobiological testing/measurementBreast malignant tumorsBreast malignancy
The invention is directed to a method of diagnosing a cell proliferative disorder of breast tissue by determining the methylation status of nucleic acids obtained from a subject. Aberrant methylation of several genes including TWIST, HOXA5, NES-1, retinoic acid receptor beta (RARβ), estrogen receptor (ER), cyclin D2, WT-1, 14.3.3 sigma, HIN-1, RASSF1A, and combinations of such genes serve as markers of breast malignancy.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Methods of diagnosing, prognosing and treating breast cancer
Owner:TEMPLE UNIVERSITY
Tumor marker STAMP-EP6 based on methylated modification
The invention provides a methylation tumor marker STAMP-EP6 and application thereof, and belongs to the field of disease diagnosis markers. The invention provides application of the methylation tumormarker STAMP-EP6 in preparing a tumor diagnosis reagent. The tumor marker STAMP-EP6 is highly methylated in all tumor types, and is lowly methylated in corresponding normal tissue, and has high sensibility and specificity, and a primer for detecting the STAMP-EP6 can be used for preparing a tumor diagnosis kit.
Owner:SHANGHAI EPIPROBE BIOTECH CO LTD
Long-chain non-coding RNA LINC01419 molecular marker of esophageal squamous cell carcinoma and application thereof
InactiveCN108588220AMicrobiological testing/measurementDNA/RNA fragmentationIndividualized treatment5 aza cdr
The invention discloses a long-chain non-coding RNA marker for assisting diagnosis of esophageal squamous cell carcinoma (ESCC). The long-chain non-coding RNA is LINC01419, the expression amount of the long-chain non-coding RNA is detected and can be used for auxiliary diagnosis or prediction of the esophageal squamous cell carcinoma, and the methylation status of a GSTP1 gene is monitored to predict the sensitivity of the esophageal squamous cell carcinoma to 5-FU chemotherapy. The expression level of a LINC01419 gene can be detected by an RT-PCR, real-time quantitative PCR, immunoassay, in situ hybridization, a chip or a high-throughput sequencing platform. The invention further discloses application of a methyltransferase inhibitor 5-Aza-CdR to regulate the expression of the long-chainnon-coding RNA LINC01419 and response to the 5-FU, and application of an inhibitor medicine including the long-chain non-coding RNA LINC01419 in treatment of the esophageal squamous cell carcinoma. The molecular marker of the esophageal squamous cell carcinoma is provided, a theoretical basis for mechanism research and clinical individualized treatment of the esophageal squamous cell carcinoma isprovided, and by monitoring the overexpression of the LINC01419 in the ESCC, a new treatment strategy for the clinical treatment of the ESCC can be provided.
Owner:汕头大学医学院附属肿瘤医院
Ribosome DNA methylation marker for detection of cancer in peripheral blood and application thereof
ActiveCN110195107AHigh copy numberTimely diagnosisMicrobiological testing/measurementDNA/RNA fragmentationCancers diagnosisRibosomal DNA
The invention provides a ribosome DNA methylation marker for detection of cancer in peripheral blood and an application thereof. The markers comprise at least one selected from the group consisting of, by taking a human ribosomal DNA repeat fragment unit reference sequence U13369.1 as a reference, CpG sites at positions of 38974, 37148, 37013, 37082, 37076, 32936, 21740, 23407, 34657, and 28277 orthe modified CpG site. The invention also provides a system, a kit, and the like for any combination of the markers for cancer diagnosis. The methylation status of the markers is significantly different between tumor tissues and non-tumor tissues, and the markers are hypomethylated in tumor tissues, and the ROC of combination of the markers in a test set to distinguish patients with liver cancer,lung cancer, and colorectal cancer can respectively reach 96%, 94%, and 92%.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com