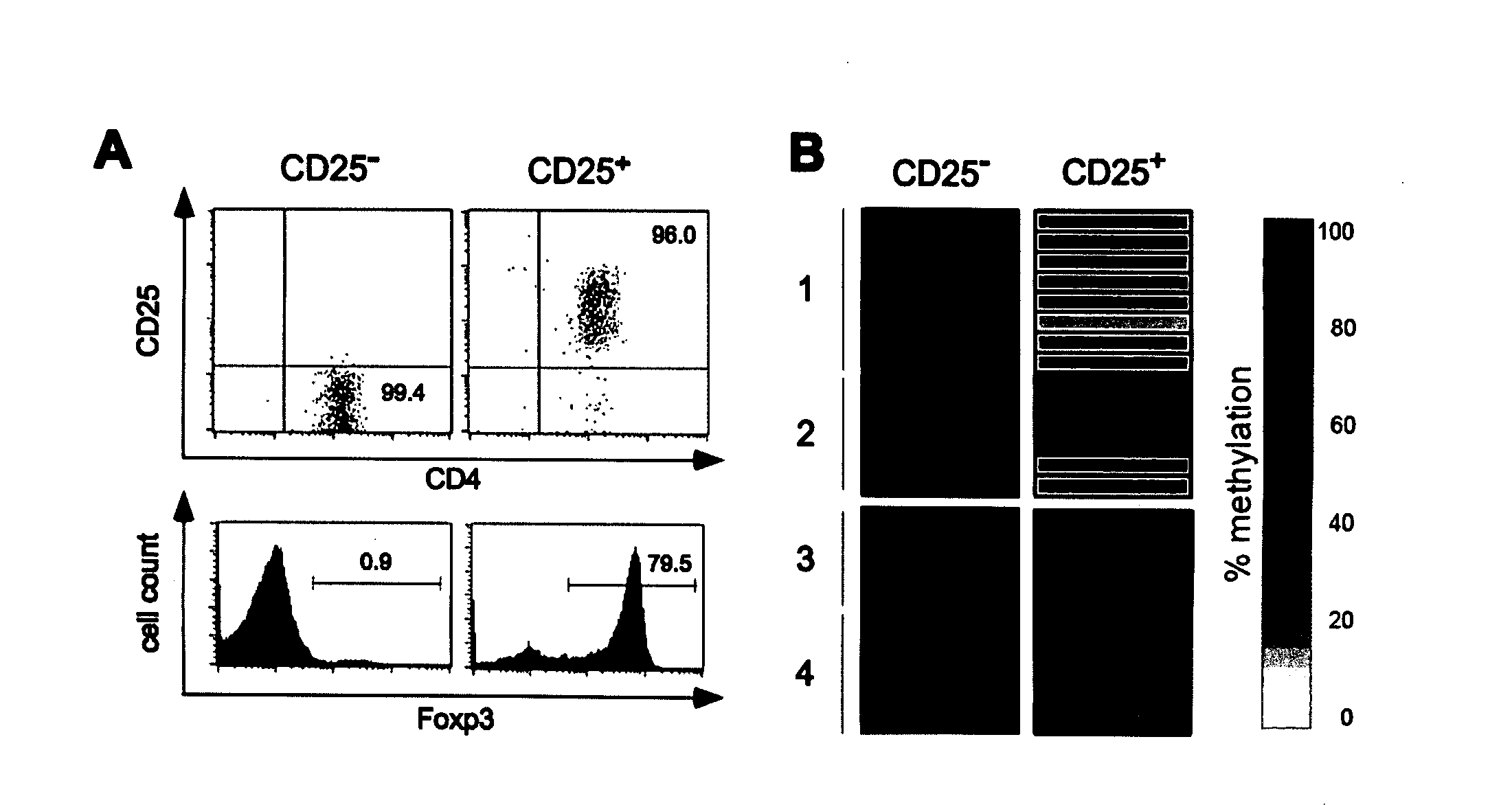
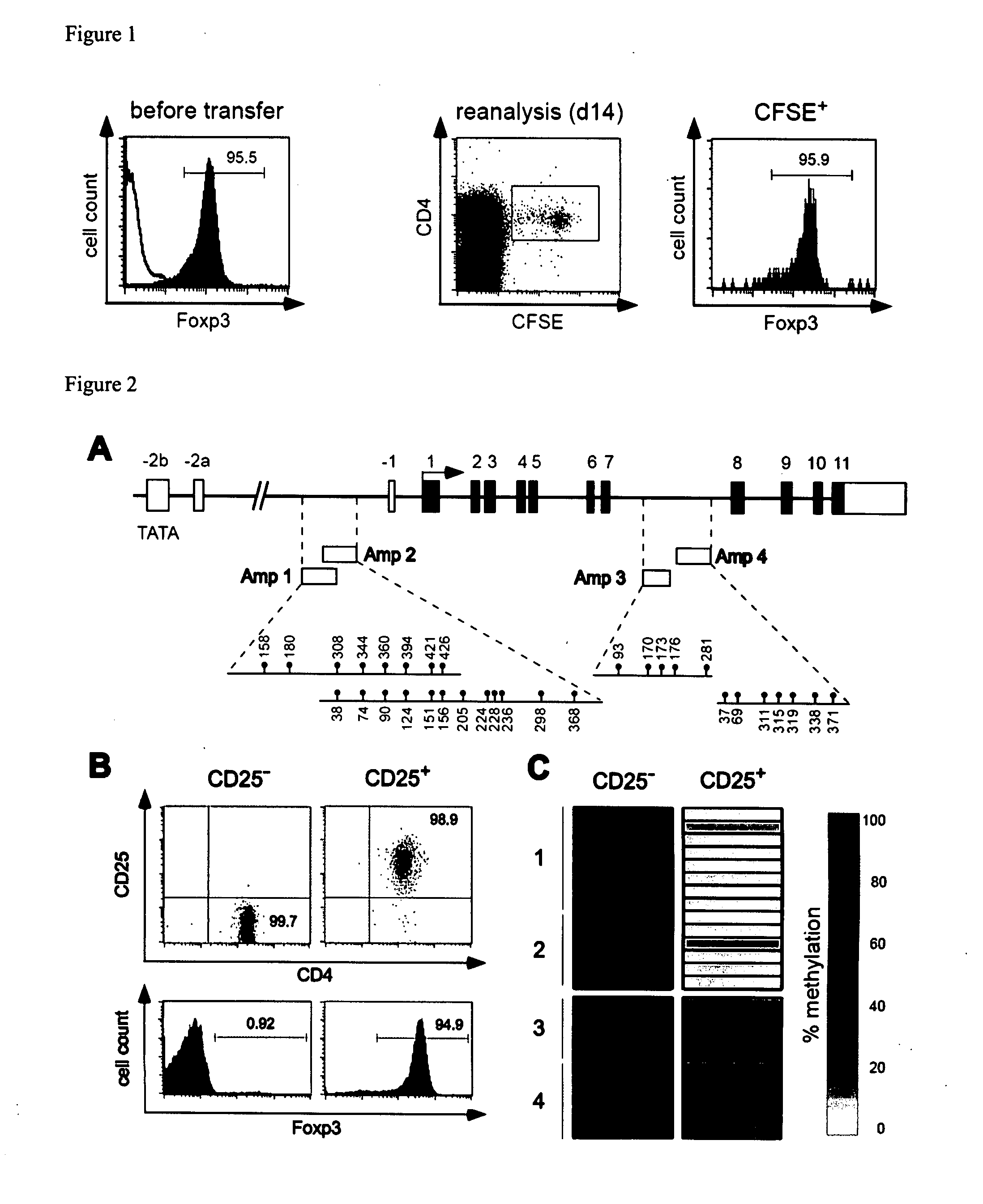
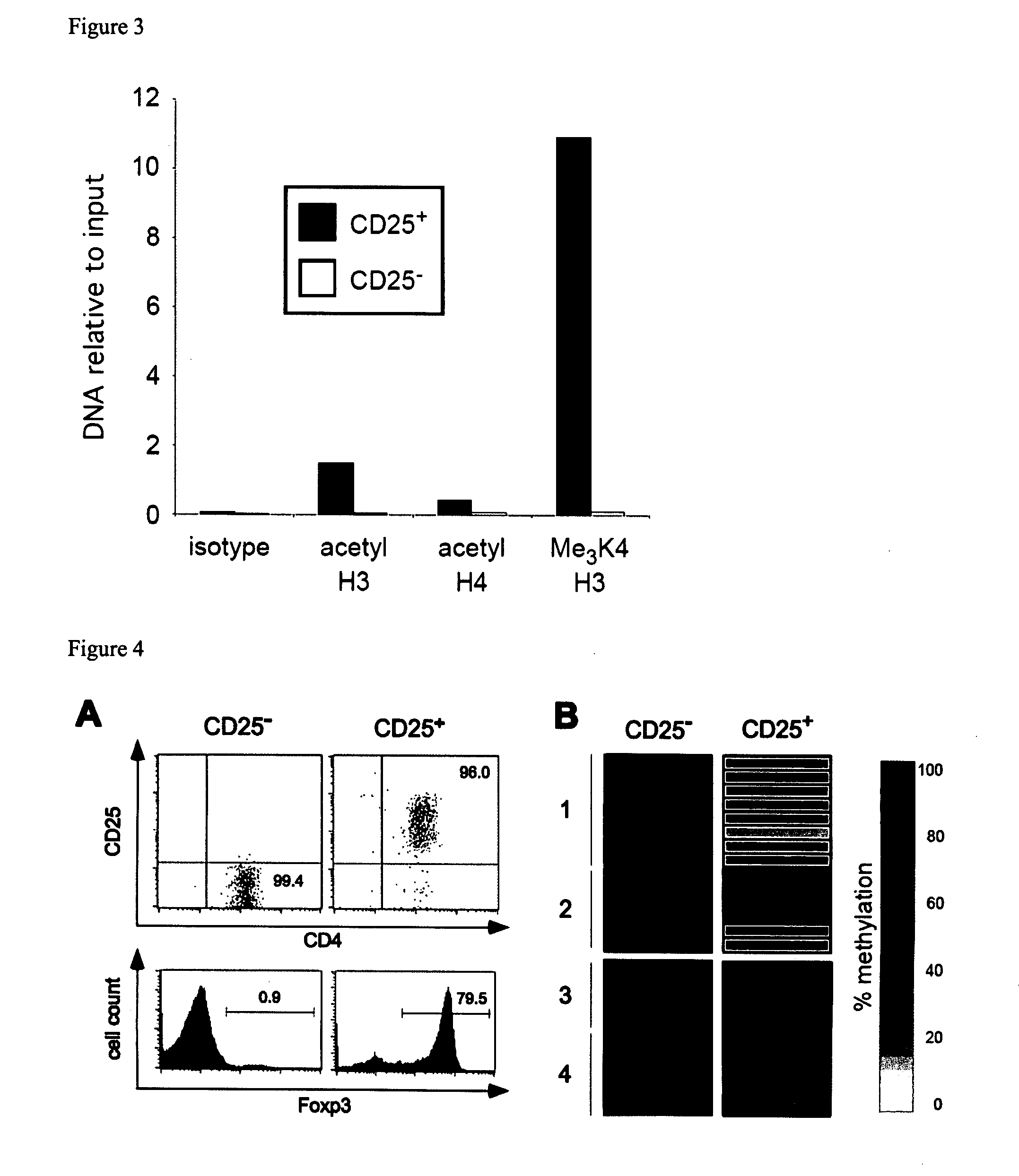
Epigenetic modification of the loci for CAMTA1 and/or FOXP3 as a marker for cancer treatment
a technology of camta1 and foxp3 is applied in the field of epigenetic modification of camta1 and/or foxp3 as a marker for cancer treatment, which can solve the problem of unfavorable prognostic indicators such as high prevalence of tregs infiltrating hcc, and achieve the effect of diagnosing the survival of a cancer patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Mice
[0086] BALB / c mice were bred at the BfR (Bundesinstitut fuer Risikobewertung, Berlin, Germany) and used at 6-12 wk of age. All animal experiments were performed under specific pathogen free conditions and in accordance with institutional, state and federal guidelines.
Antibodies, Staining Reagents and Cytokines
[0087] The following antibodies were produced in the inventors' laboratory: anti FcR II / III (2.4G2), anti CD4 (GK1.5), anti CD3 (145.2C11), anti CD28 (37.51) and anti IL-4 (11B11). The following antibodies and secondary reagents were purchased from BD PharMingen: anti CD4 (RM4-5), anti CD19 (1D3), anti CD25 (7D4), anti CD8 (53-6.7), anti CD25 (PC6.1), anti CD62L (Mel-14), streptavidin, and appropriate isotype controls. The PE anti-mouse Foxp3 staining set was purchased from eBioscience. All microbeads were obtained from Miltenyi Biotec and all cytokines from RandD systems.
[0088] Cytometric analysis was performed as previously described (Huehn, J. et al...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| chromatin structure | aaaaa | aaaaa |
| current state | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com