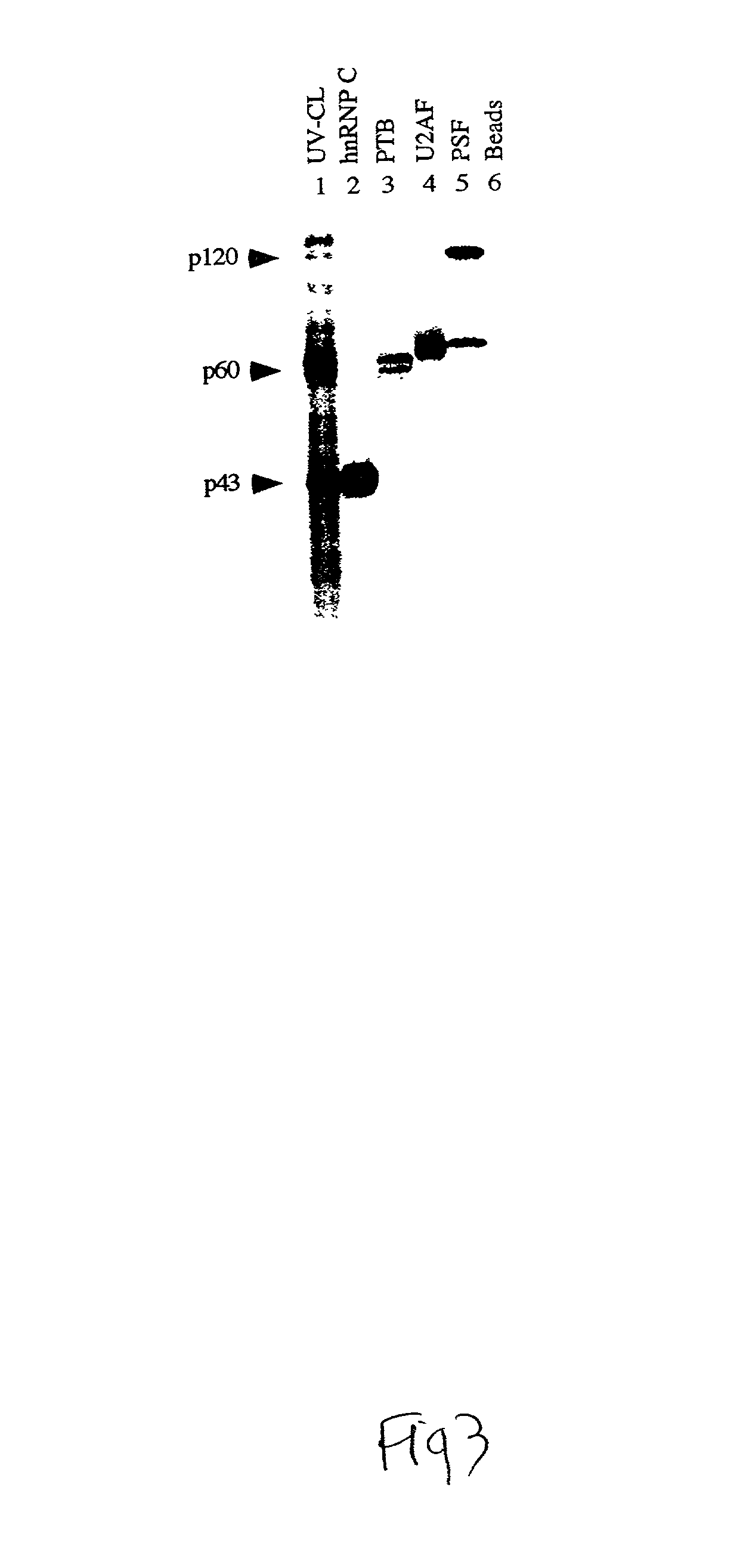
Patents
Literature
58 results about "Disease outcome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The condition of a patient at the end of therapy or a disease process, including the degree of wellness and the need for continuing care, medication, support, counseling, or education. outcome. A result, new condition or event occurring in individual study subjects which is used to assess efficacy. Outcomes measure.
Method for cancer detection, diagnosis and prognosis
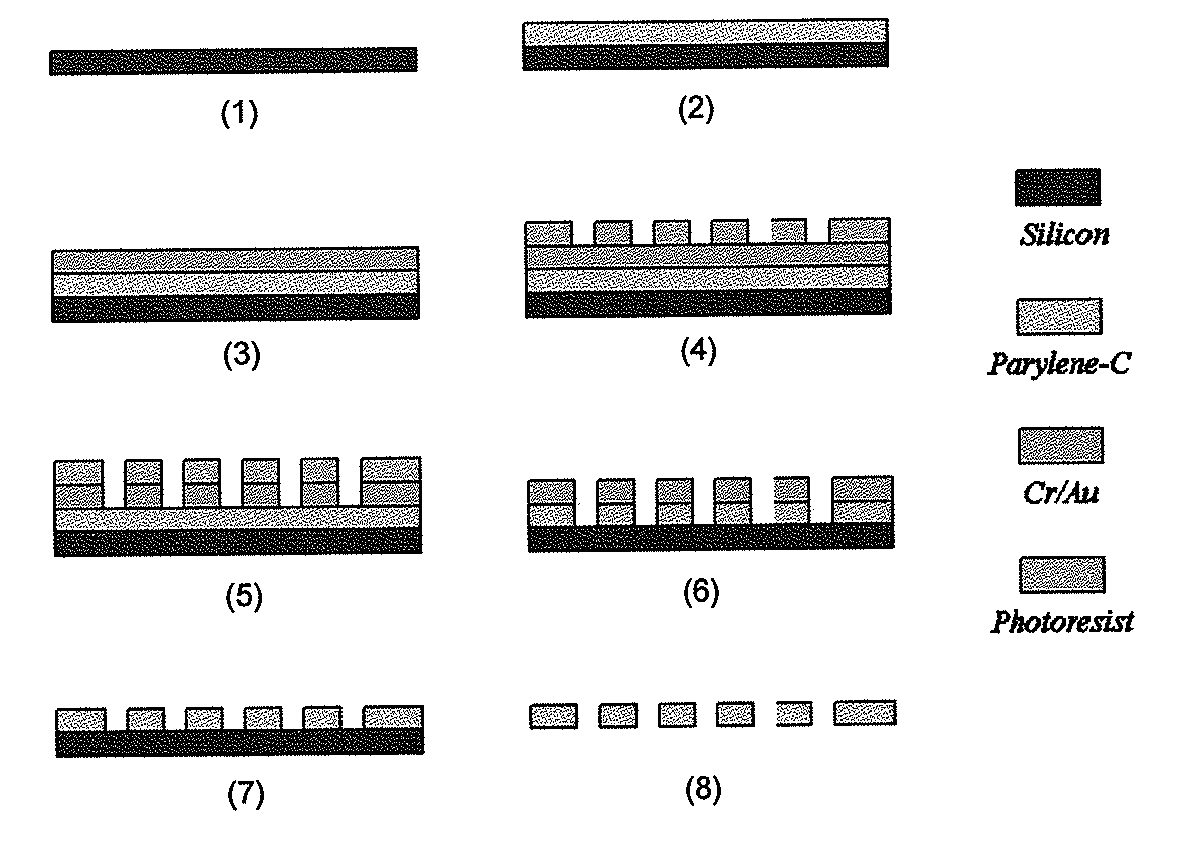
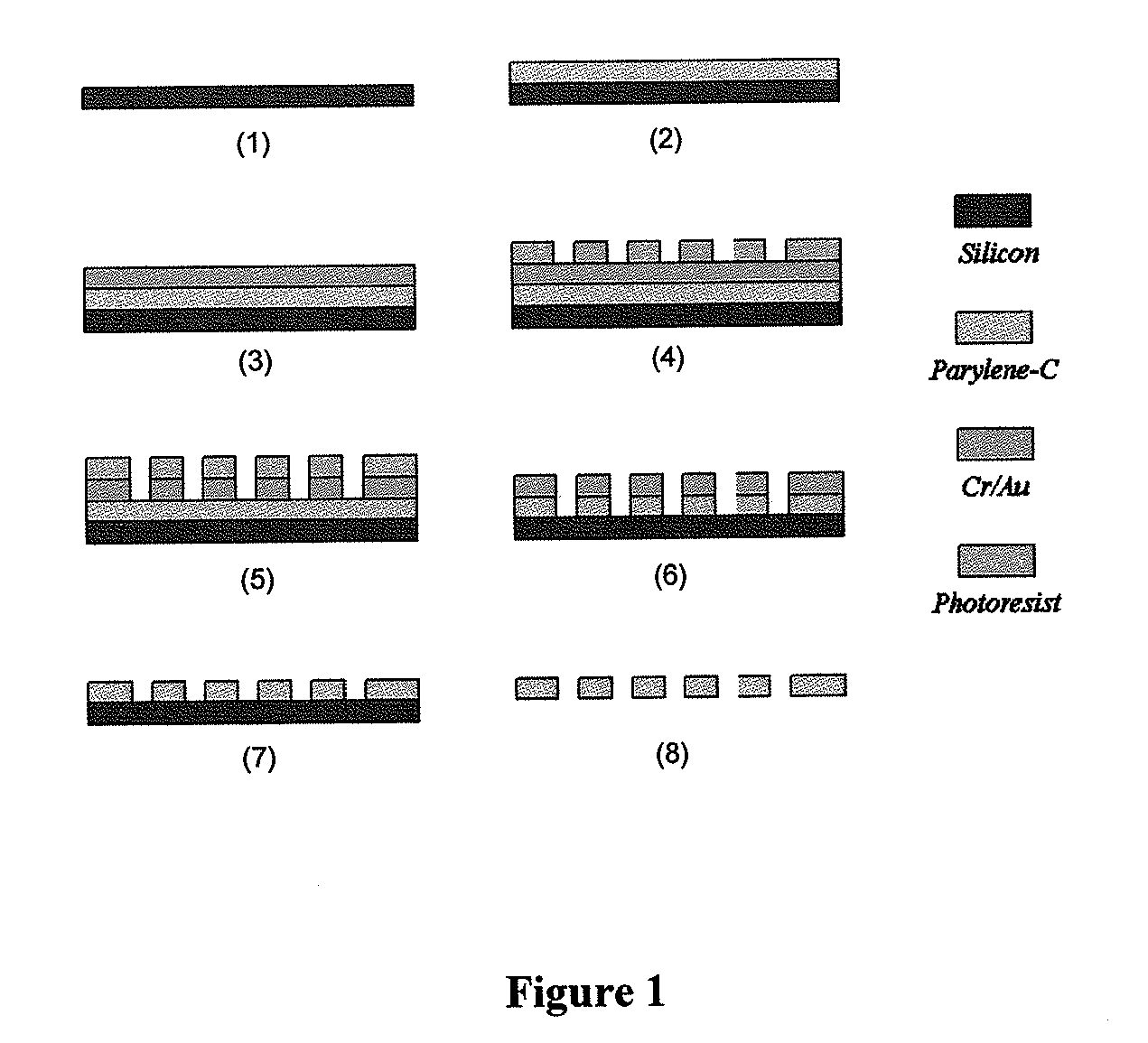
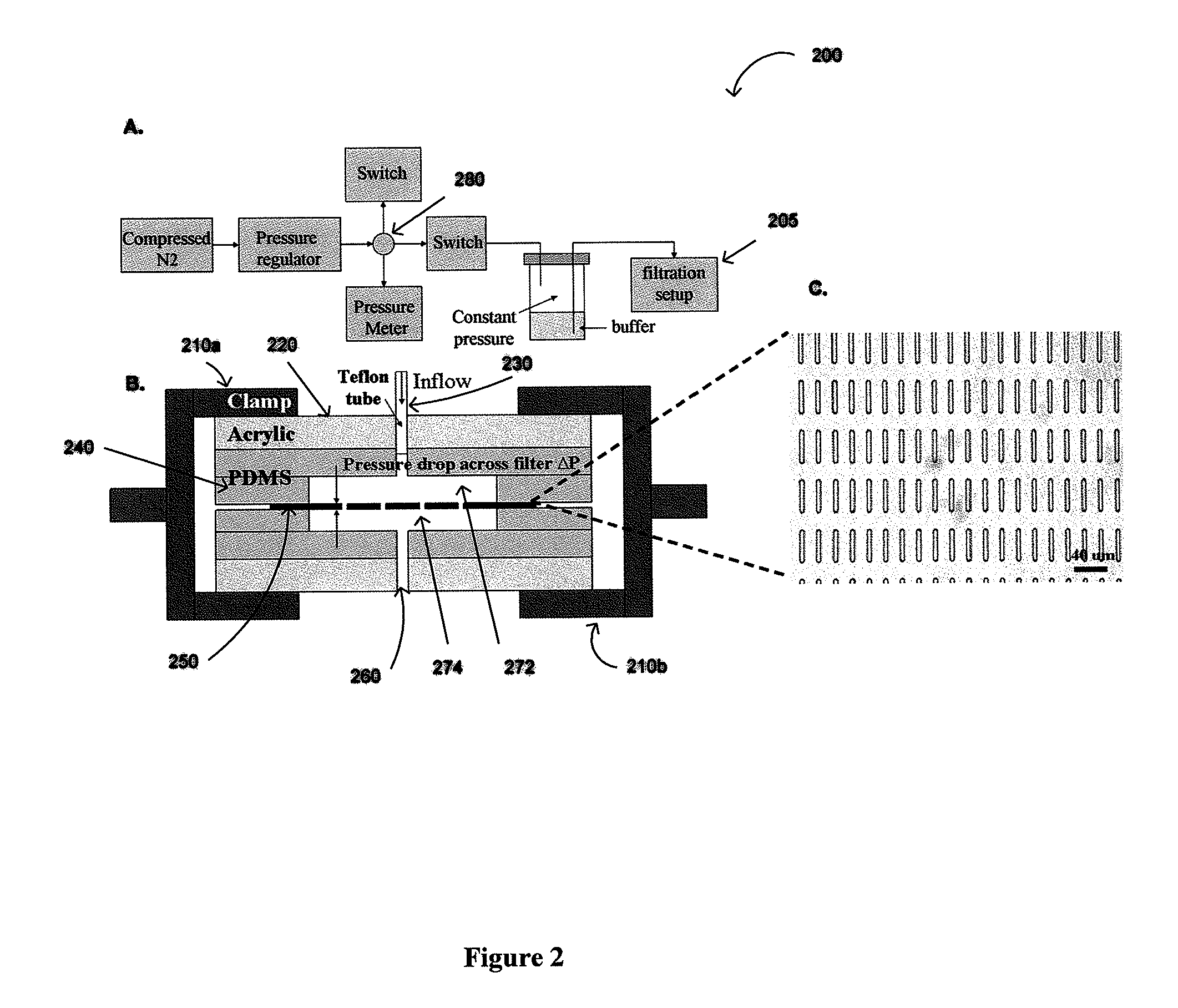
ActiveUS20110053152A1Rapidly and efficiently captureImprove capture efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsTelomeraseParylene
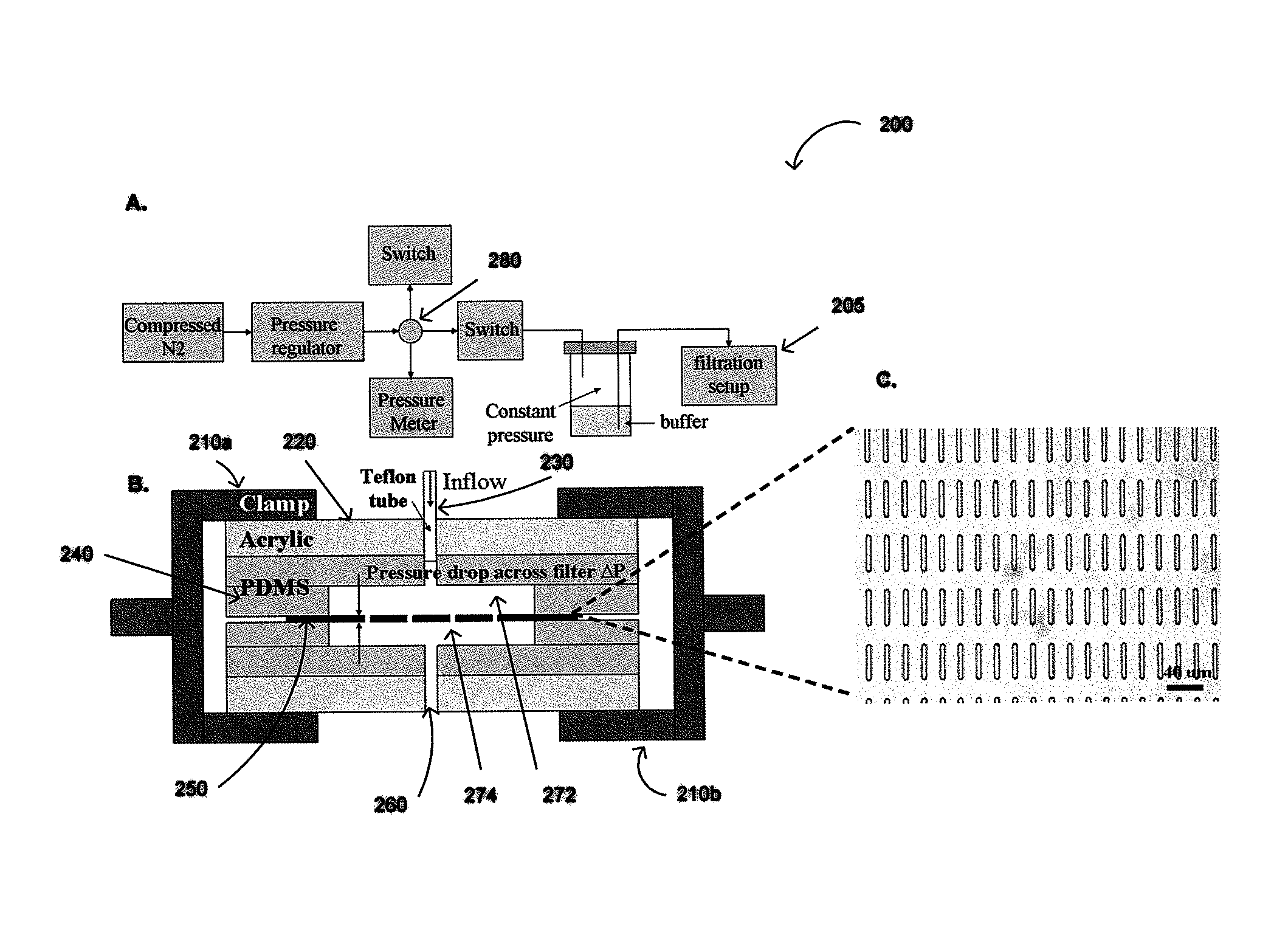
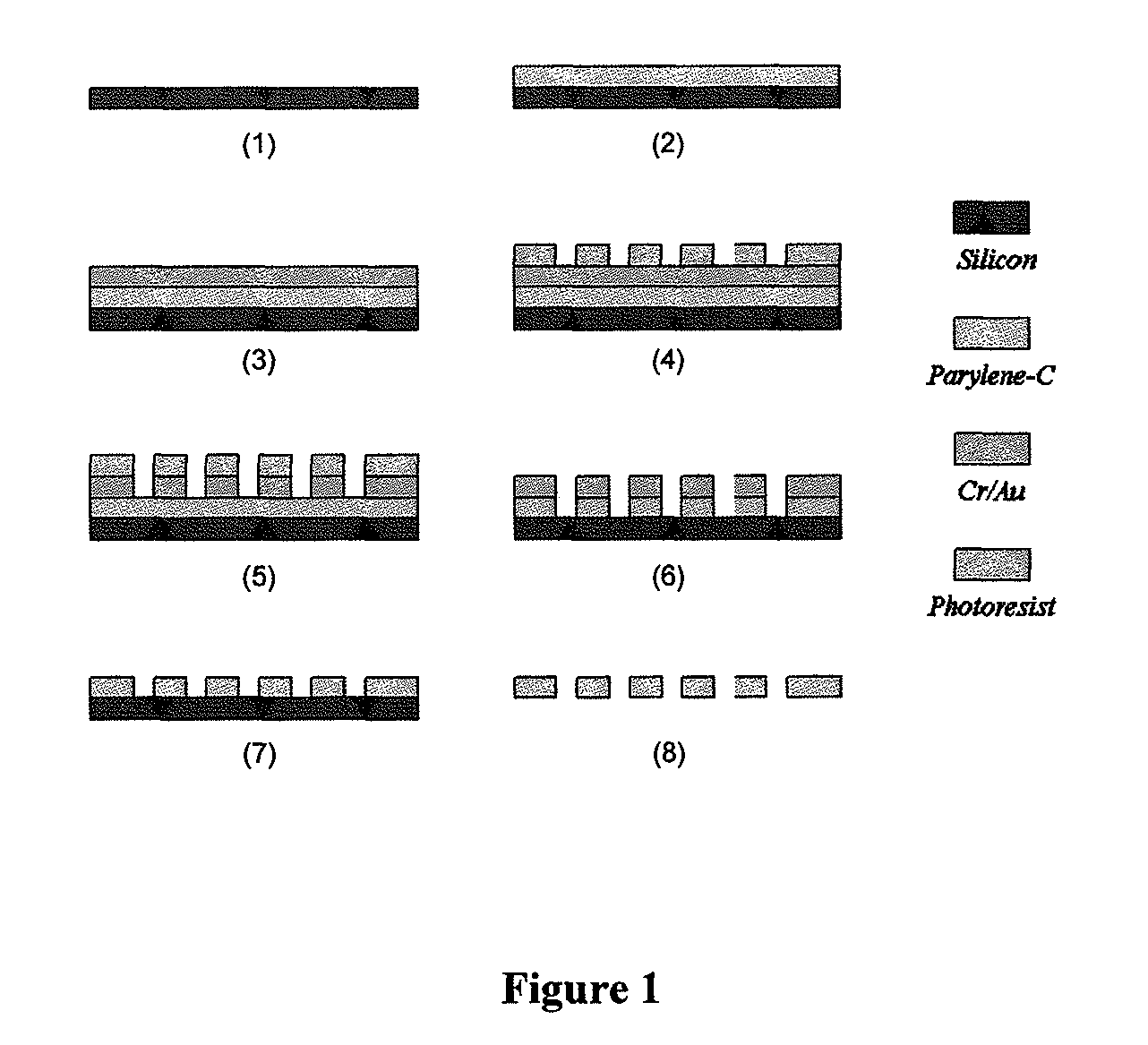
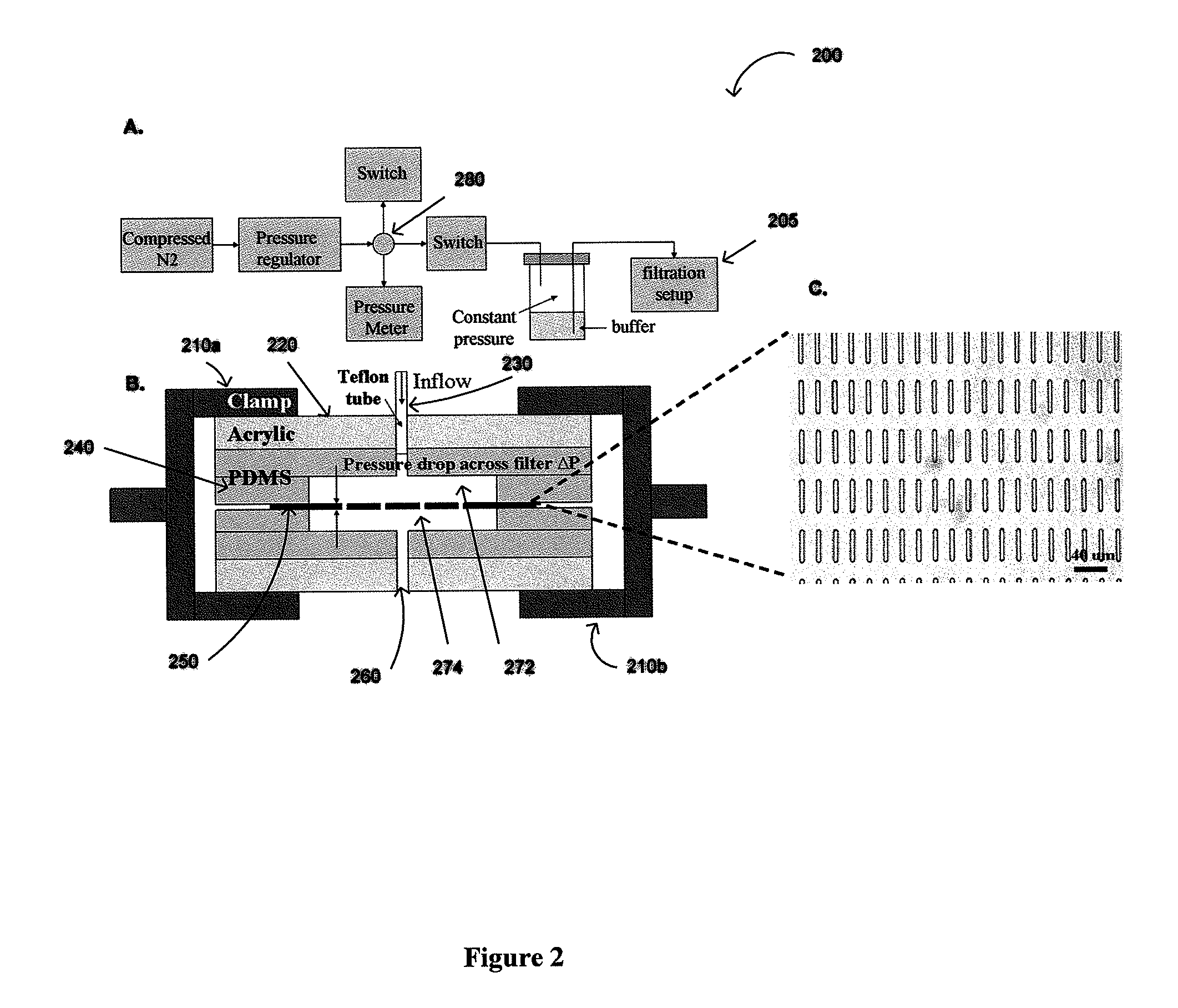
The present invention provides a method for diagnosing cancer, predicting a disease outcome or response to therapy in a patient sample. The method involves isolating a circulating tumor cell (CTC), for example, a viable CTC, from a sample using a parylene microfilter device comprising a membrane filter having or consisting of a parylene substrate, which has an array of holes with a predetermined shape and size; and detecting and quantifying telomerase activity in blood circulating tumor cells. The invention further provides methods of using cells live-captured in various applications.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Modeling of systemic inflammatory response to infection
InactiveUS20070083333A1Microbiological testing/measurementBiostatisticsDisease outcomeBiomarker panel
Models for the systemic inflammatory response to infection, which involve the use of immunocompromised animals, and methods of using the models are described. These models can be used in identifying analytes or biomarker panels that can be used in staging or monitoring sepsis. The models can also be used for predicting an animal's disease outcome or in providing a prognosis for sepsis patients. Further, the invention relates to methods for evaluating potential treatments for sepsis.
Owner:JANSSEN PHARMA NV
Marker of prostate cancer
InactiveUS20100303795A1Increasing transcriptionalImprove translationOrganic active ingredientsSugar derivativesDisease outcomeProstate cancer
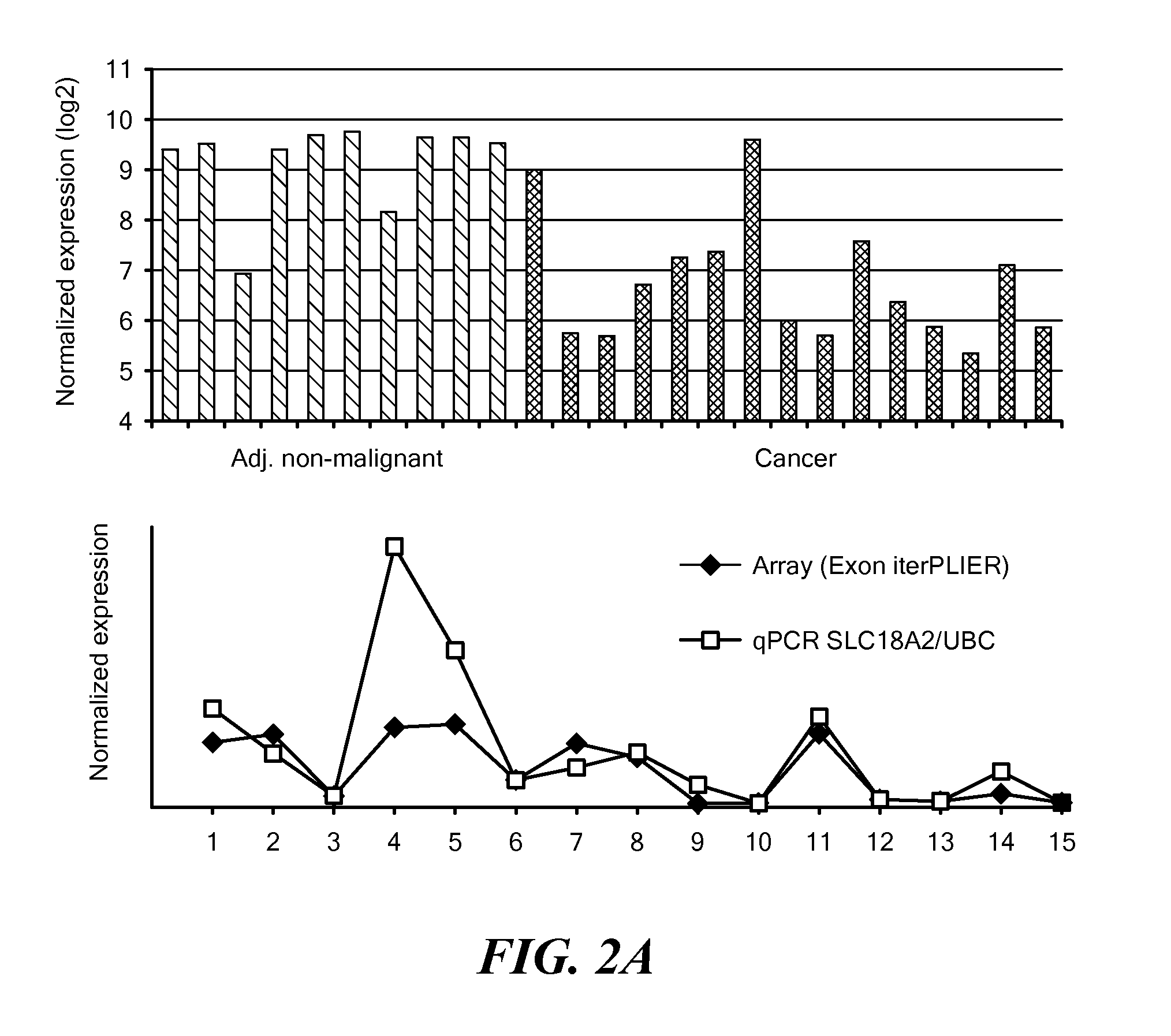
An SLC18A2 gene serves as a marker of prostate cancer. Methods are provided for diagnosing prostate cancer, predicting or prognosticating the disease outcome, predicting recurrence following surgery, and monitoring disease progression in an individual having prostate cancer. The methods relate to determining the methylation state of an SLC18A2 gene and / or determining the level of transcription or translation of the gene in a sample from the individual. Methods of treating prostate cancer are also provided. The invention also pertains to compositions and kits for use in the methods.
Owner:AARHUS UNIV +1
Method for cancer detection, diagnosis and prognosis
ActiveUS8551425B2Rapidly and efficiently captureImprove capture efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsTelomeraseParylene
The present invention provides a method for diagnosing cancer, predicting a disease outcome or response to therapy in a patient sample. The method involves isolating a circulating tumor cell (CTC), for example, a viable CTC, from a sample using a parylene microfilter device comprising a membrane filter having or consisting of a parylene substrate, which has an array of holes with a predetermined shape and size; and detecting and quantifying telomerase activity in blood circulating tumor cells. The invention further provides methods of using cells live-captured in various applications.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Detection kit for schistosomiasis japonica blood serum designated object
InactiveCN101393217AImprove featuresIncreased sensitivityMaterial analysisPeptide antigenTherapeutic effect
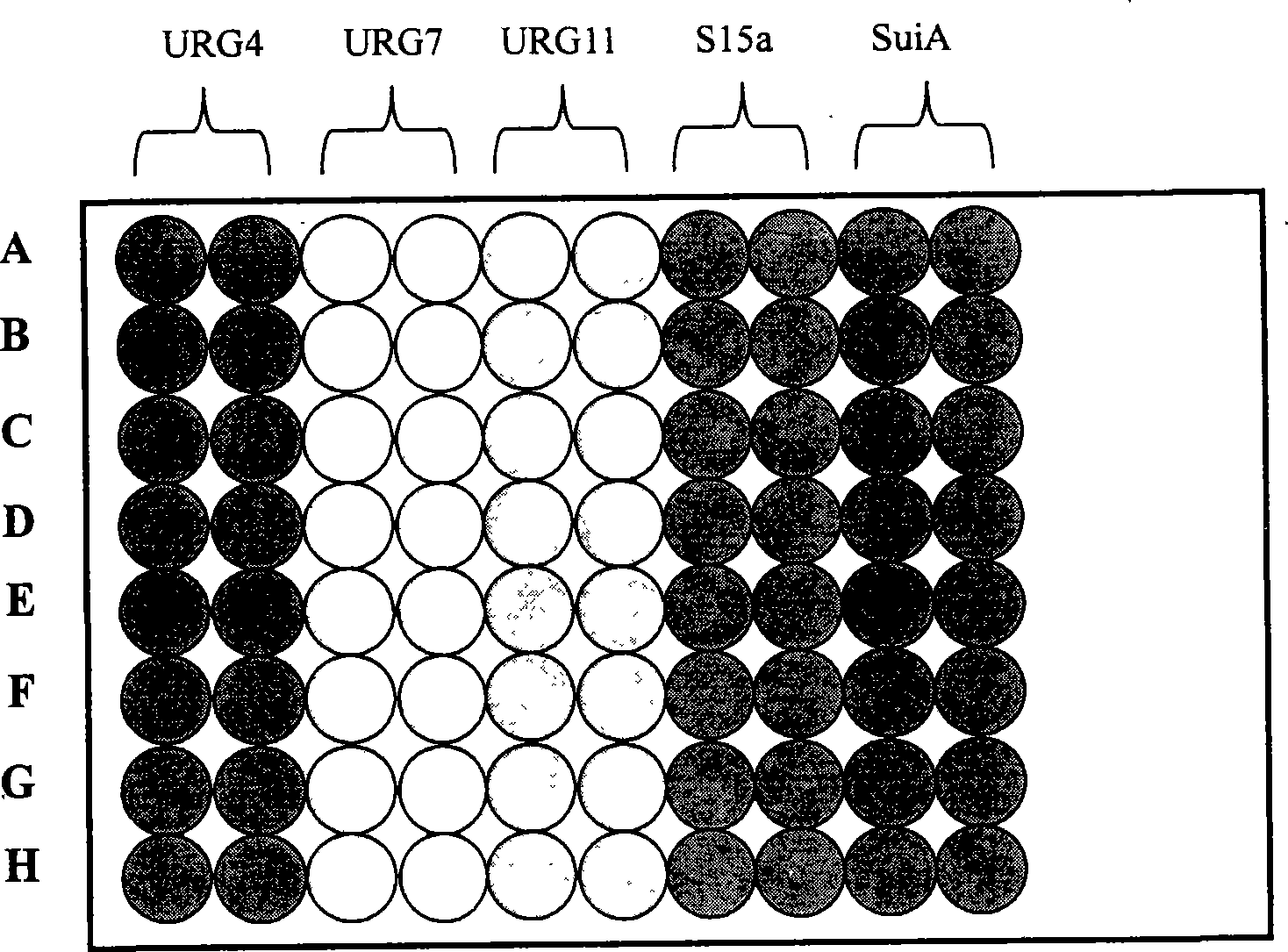
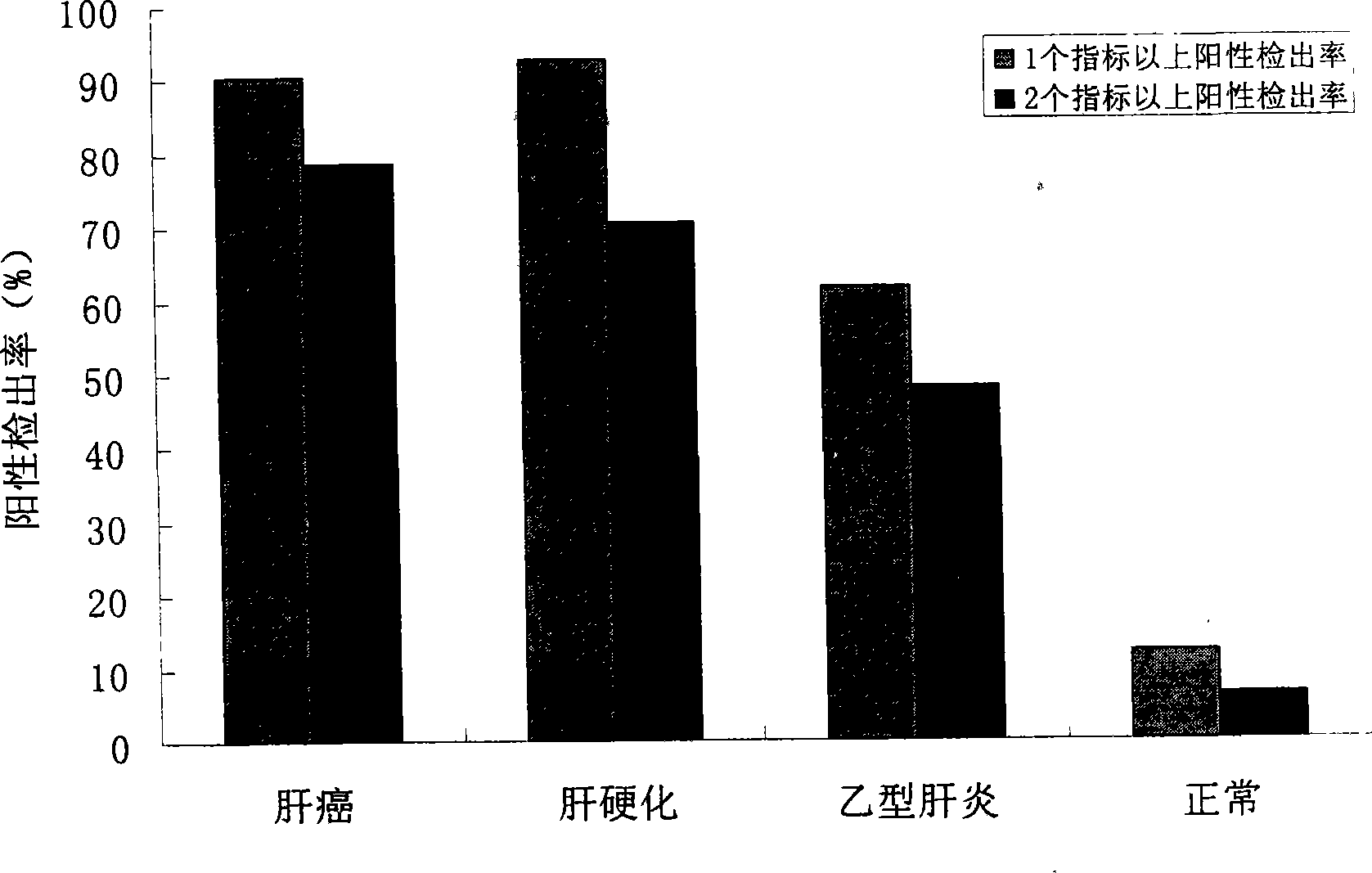
The invention relates to the technical field of biomedical diagnostics and discloses a reagent kit for detecting a serum marker of primary hepatic carcinoma, which consists of an ELISA plate enveloped by antigens, an enzyme-labeled antibody working solution, a sample diluent, a washing liquid, positive and negative control serum, a chromogenic solution and a stopping liquid. The reagent kit is characterized in that the envelope antigens of the ELISA plate are five groups of peptide antigens L4-A and L4-B; L7-B; L11-1, L11-3 and L11-4; L12-A and L12-B; and Sui1-A and Sui1-B, which correspond to five serum marker antibodies of the primary hepatic carcinoma; and Sui1-A and Sui1-B respectively. Except the L7B which is separately enveloped, each group of all the other groups of peptide antigens is enveloped by proportionally mixing polypeptides in the same group. Proven by evaluation experiments of the reagent kit and clinical trial, the reagent kit has good specificity and sensitivity, and can be used for early warning prompt or early diagnosis before the primary hepatic carcinoma appears or at early stage of the primary hepatic carcinoma, thereby improving the survival rate of carcinoma patients and evaluating treatment effect and disease outcome in time by observing the dynamic change of related indicators of the serum.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
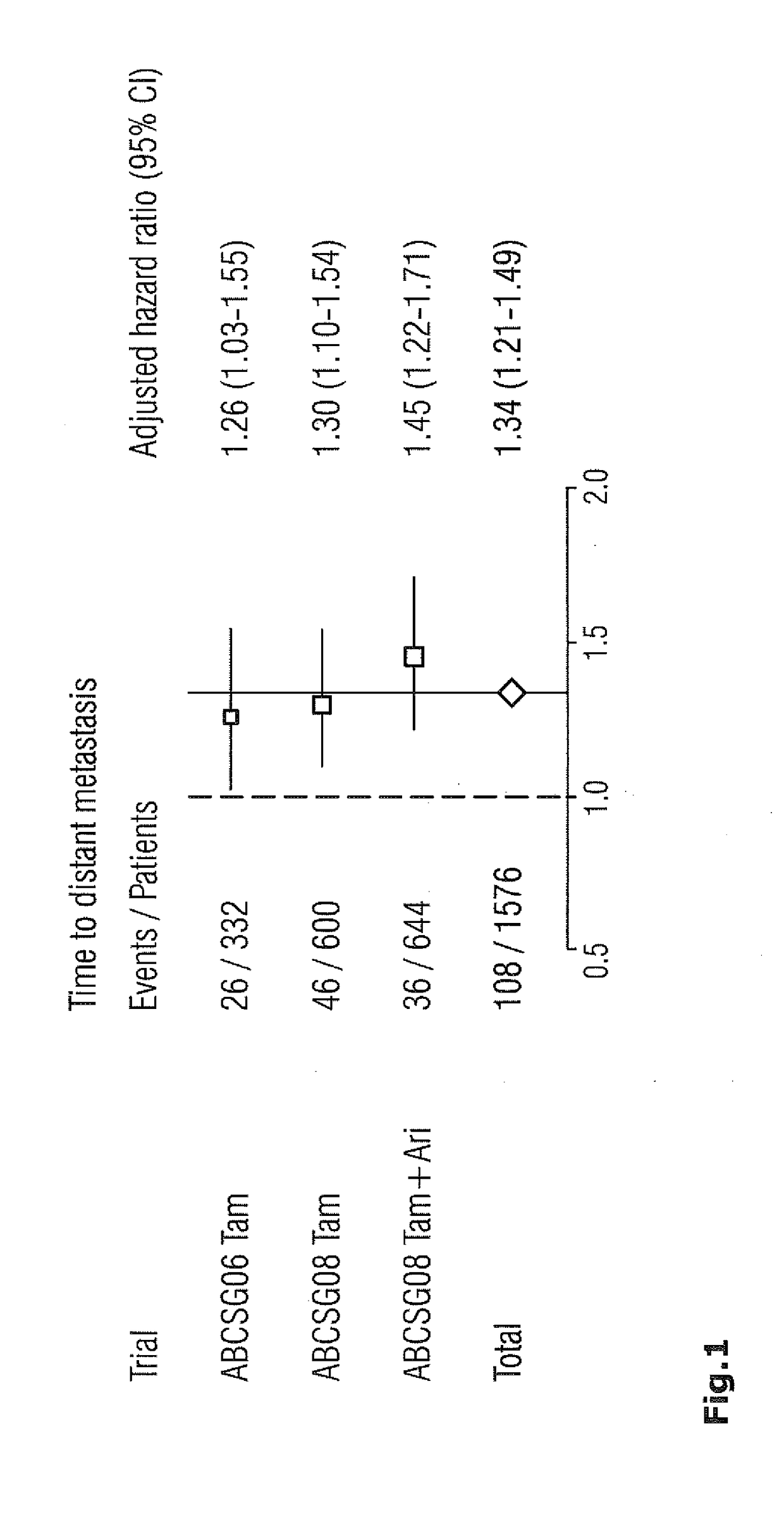
Method for breast cancer recurrence prediction under endocrine treatment
InactiveUS20130065786A1Reliable predictionNucleotide librariesMicrobiological testing/measurementDisease outcomeEndocrine therapy
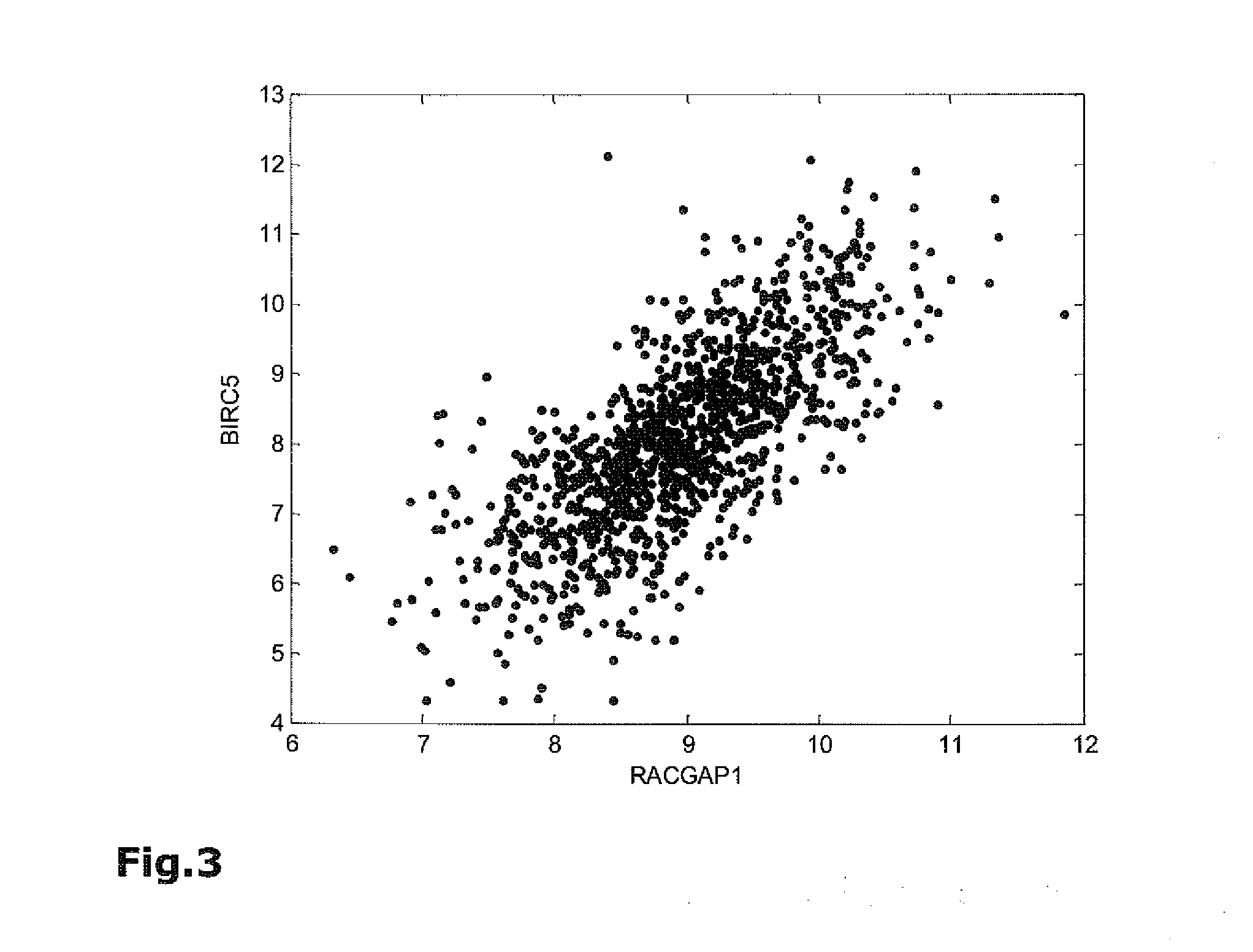
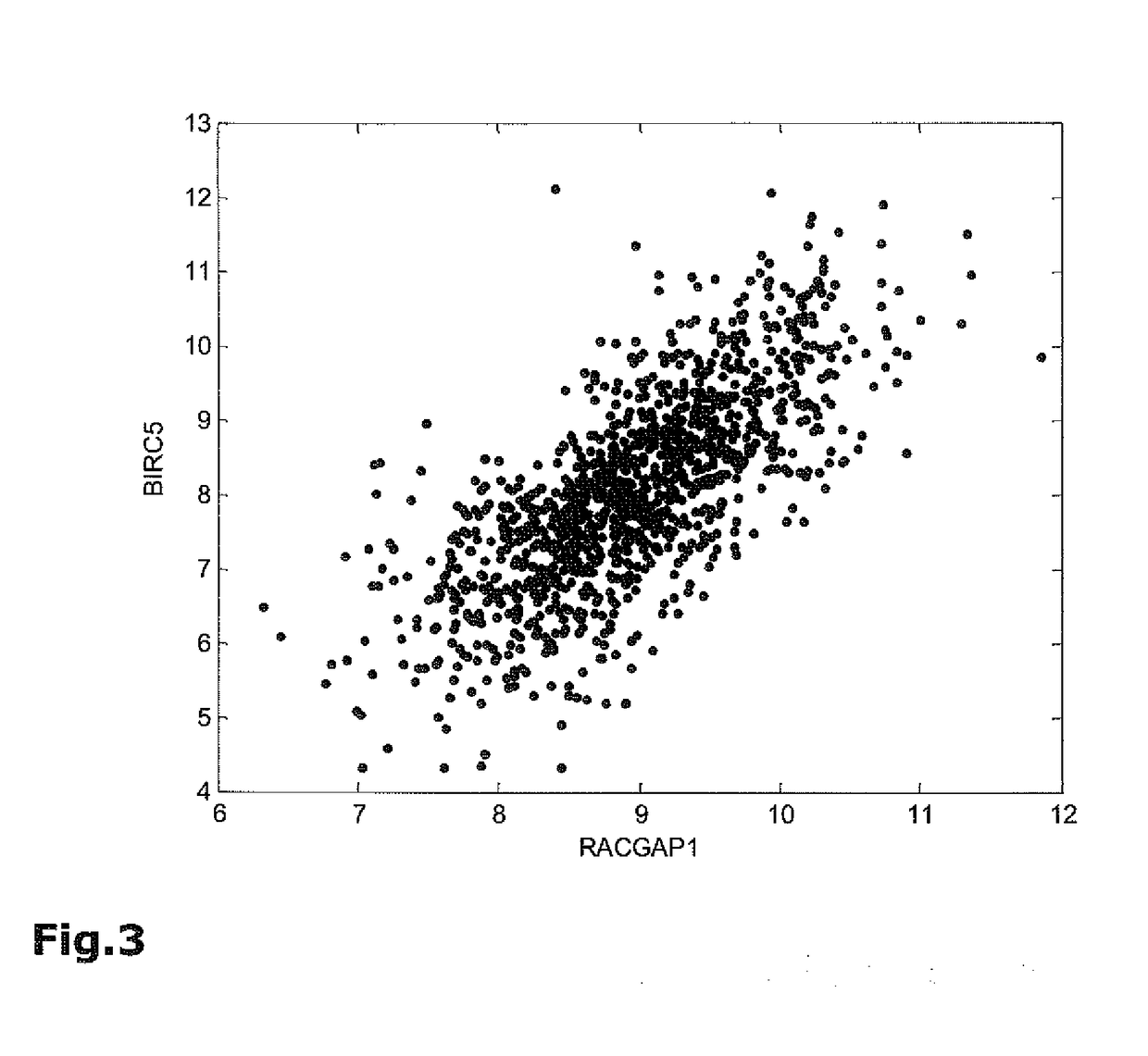
The present invention relates to methods, kits and systems for the prognosis of the disease outcome of breast cancer, said method comprising:(a) determining in a tumor sample from said patient the RNA expression levels of at least 2 of the following 9 genes: UBE2C, BIRC5, RACGAP1, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP(b) mathematically combining expression level values for the genes of the said set which values were determined in the tumor sample to yield a combined score, wherein said combined score is indicative of a prognosis of said patient; and kits and systems for performing said method.
Owner:SIVIDON DIAGNOSTICS
A new classifier for the molecular classification of multiple myeloma
ActiveUS20140221313A1Conducive to survivalPoor survival rateBiocideMicrobiological testing/measurementDisease outcomeMolecular classification
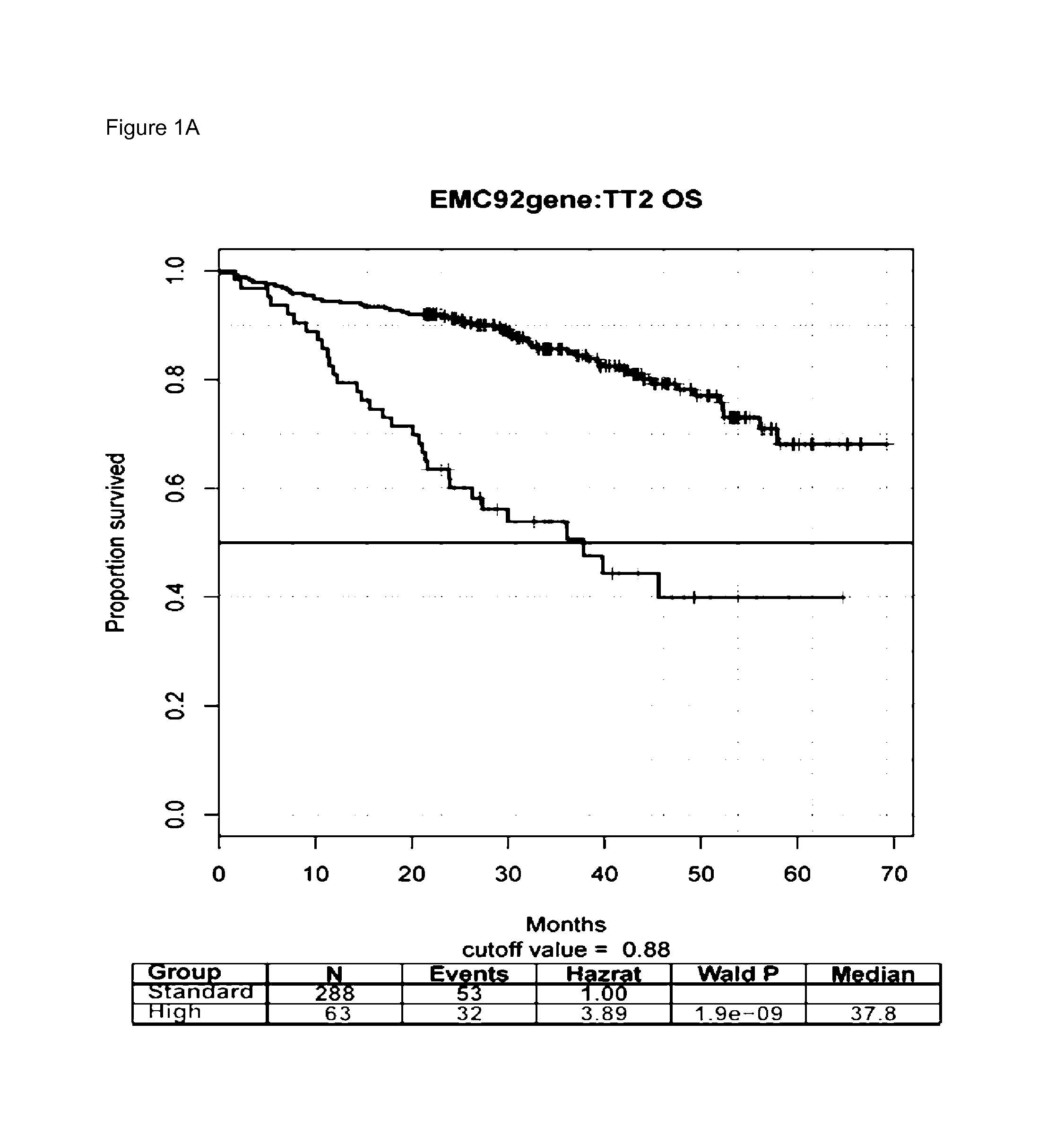
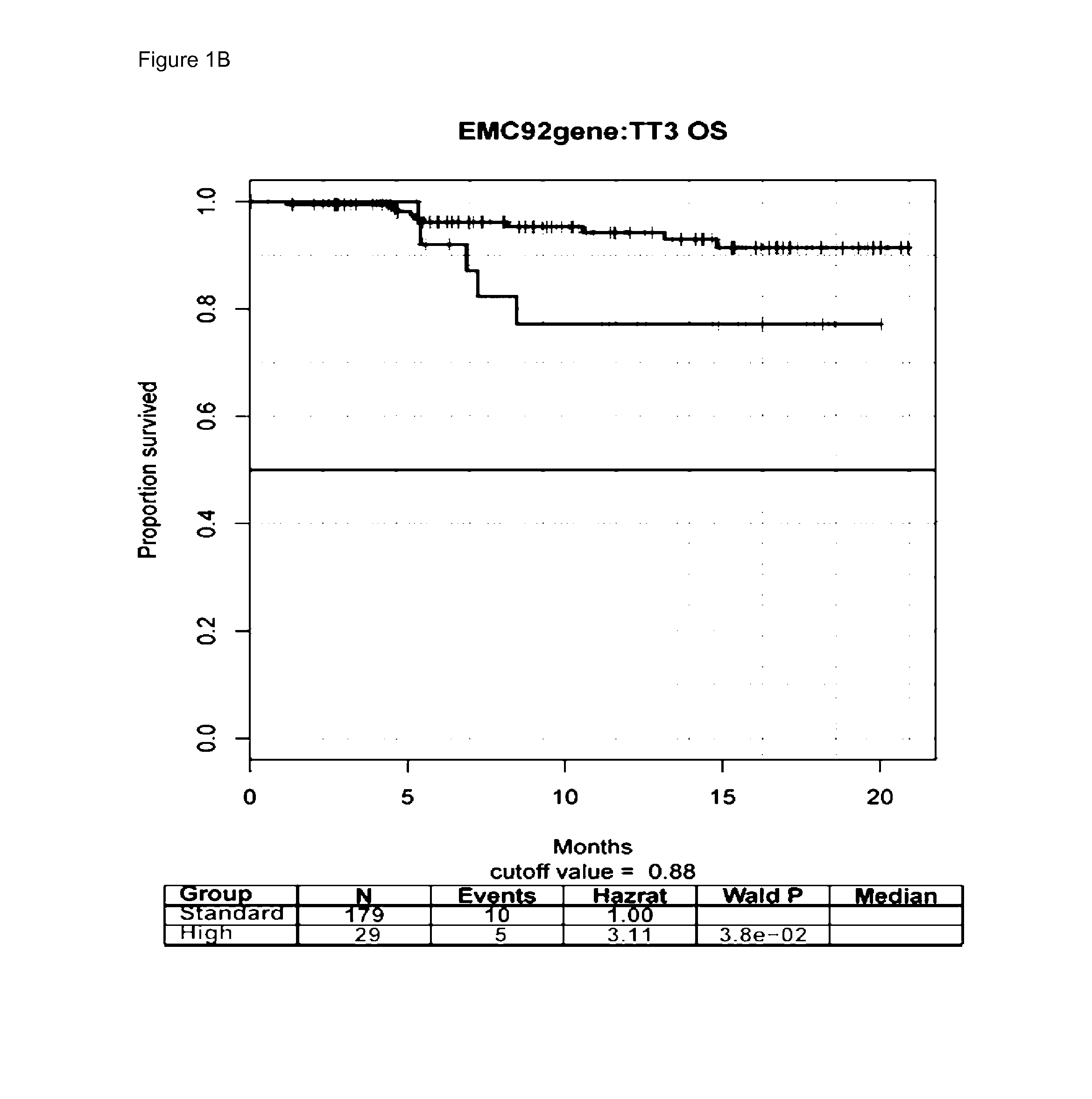
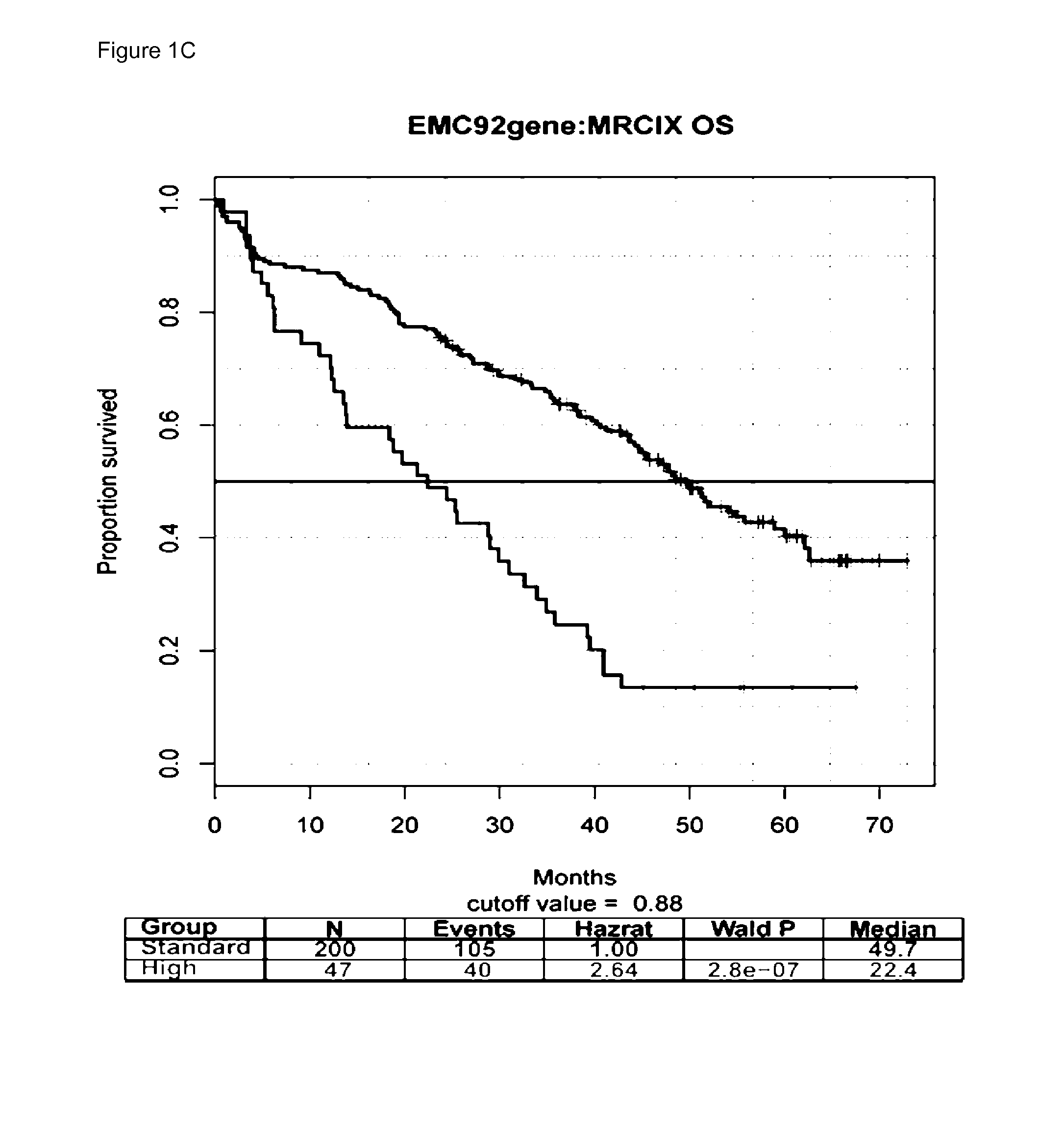
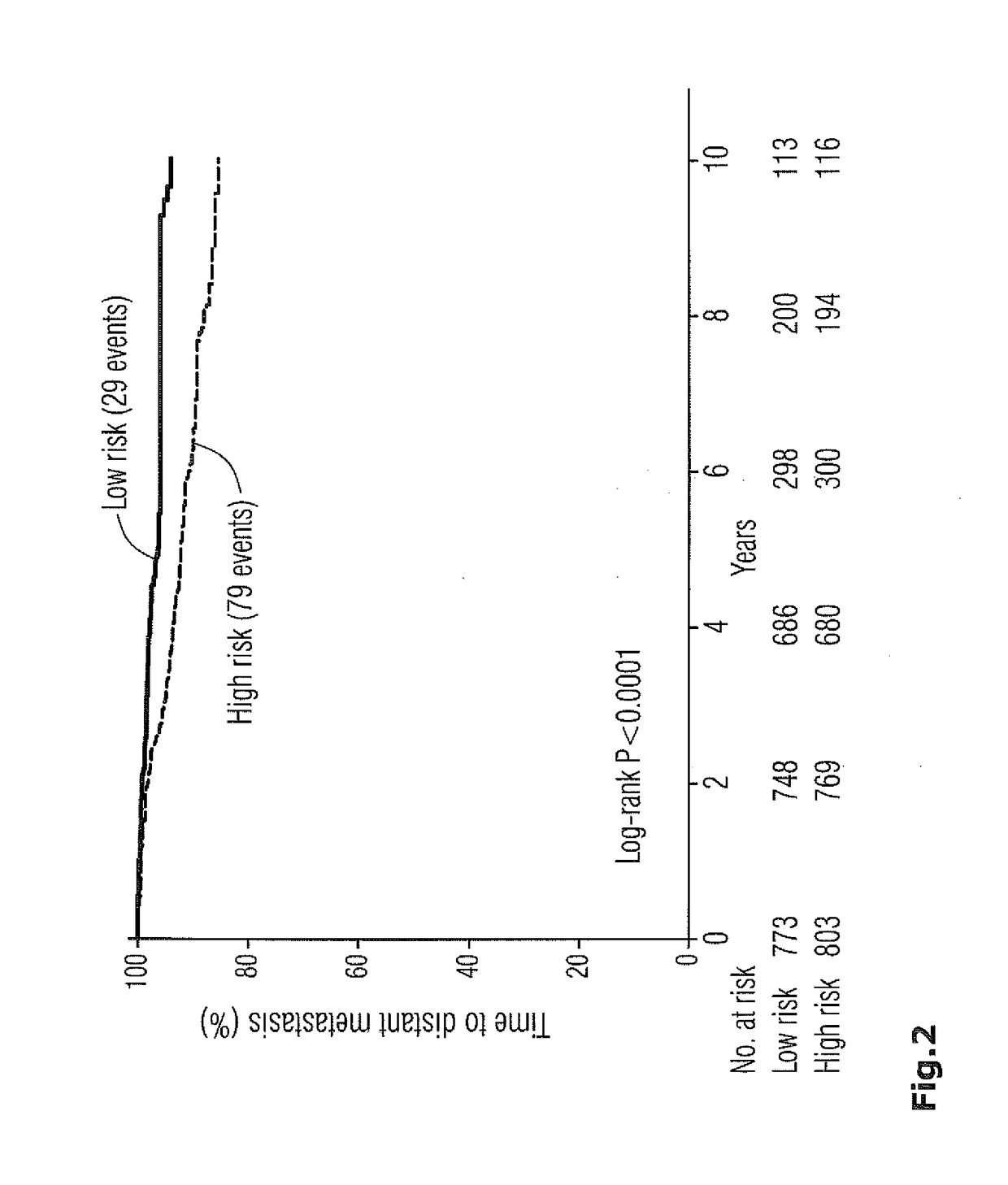
The present invention is in the field of molecular diagnostics and relates to a method for classifying samples obtained from patients diagnosed with multiple myeloma into three newly defined clusters. The invention also relates to a method for determining the prognosis of an individual diagnosed with multiple myeloma as well as a method for the prediction of the response to treatment of an individual diagnosed with multiple myeloma. More in particular, the invention provides a method for determining the disease outcome or the prognosis of a patient diagnosed with multiple myeloma by classifying said patient into a high risk or a low risk category, based on a 92 gene classifier.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Methods for Breast Cancer Prognosis
InactiveUS20090311700A1Accurate predictionBioreactor/fermenter combinationsBiological substance pretreatmentsDisease outcomeNode negative
The present invention relates to methods, kits and systems for the prognosis of the disease outcome of breast cancer in untreated breast cancer patients. More specific, the present invention relates to the prognosis of breast cancer based on measurements of the expression levels of marker genes in tumor samples of breast cancer patients. Marker genes are disclosed which allow for an accurate prognosis of breast cancer in patients having node negative, fast proliferating breast cancer.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Methods and kits used in identifying glioblastoma
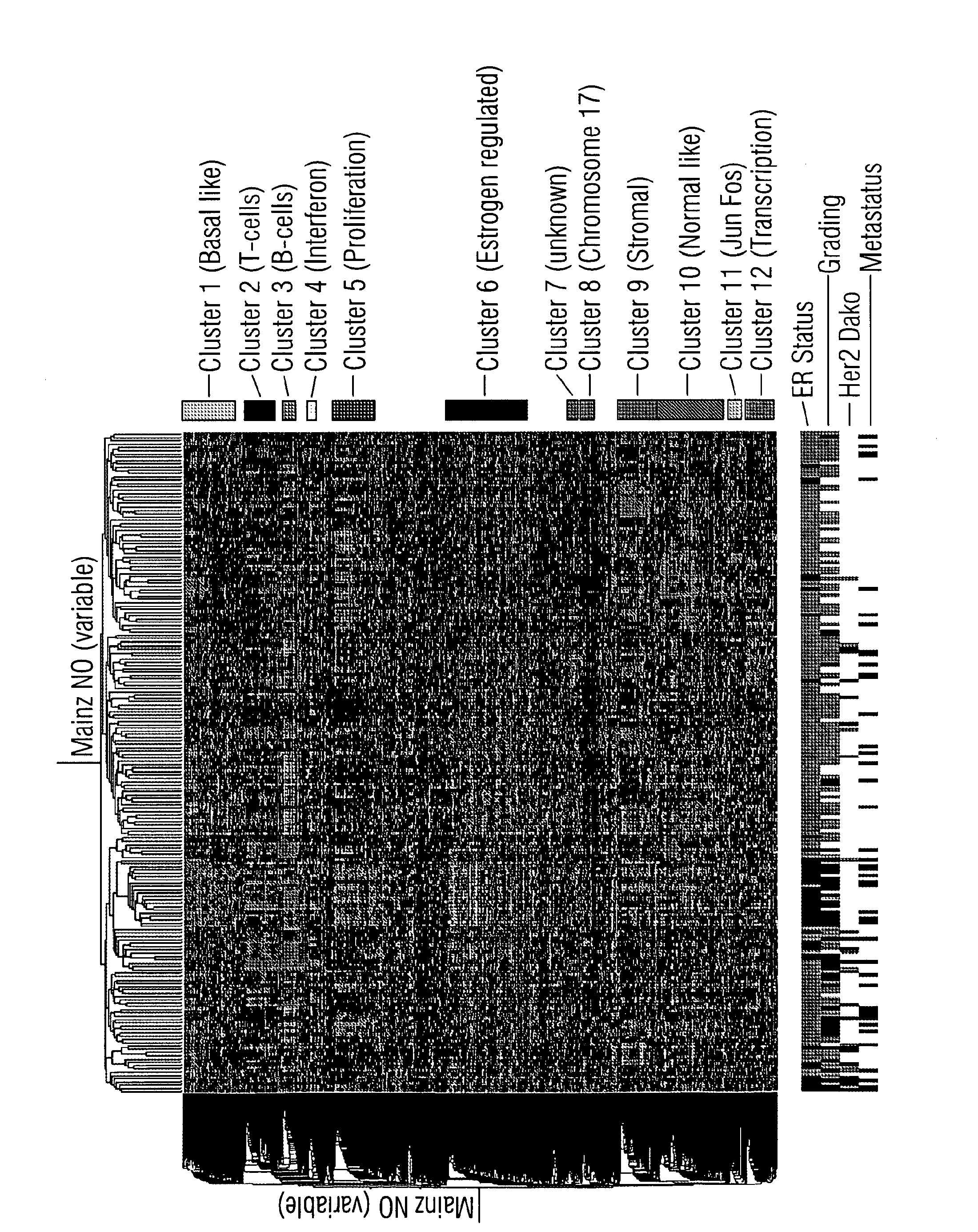
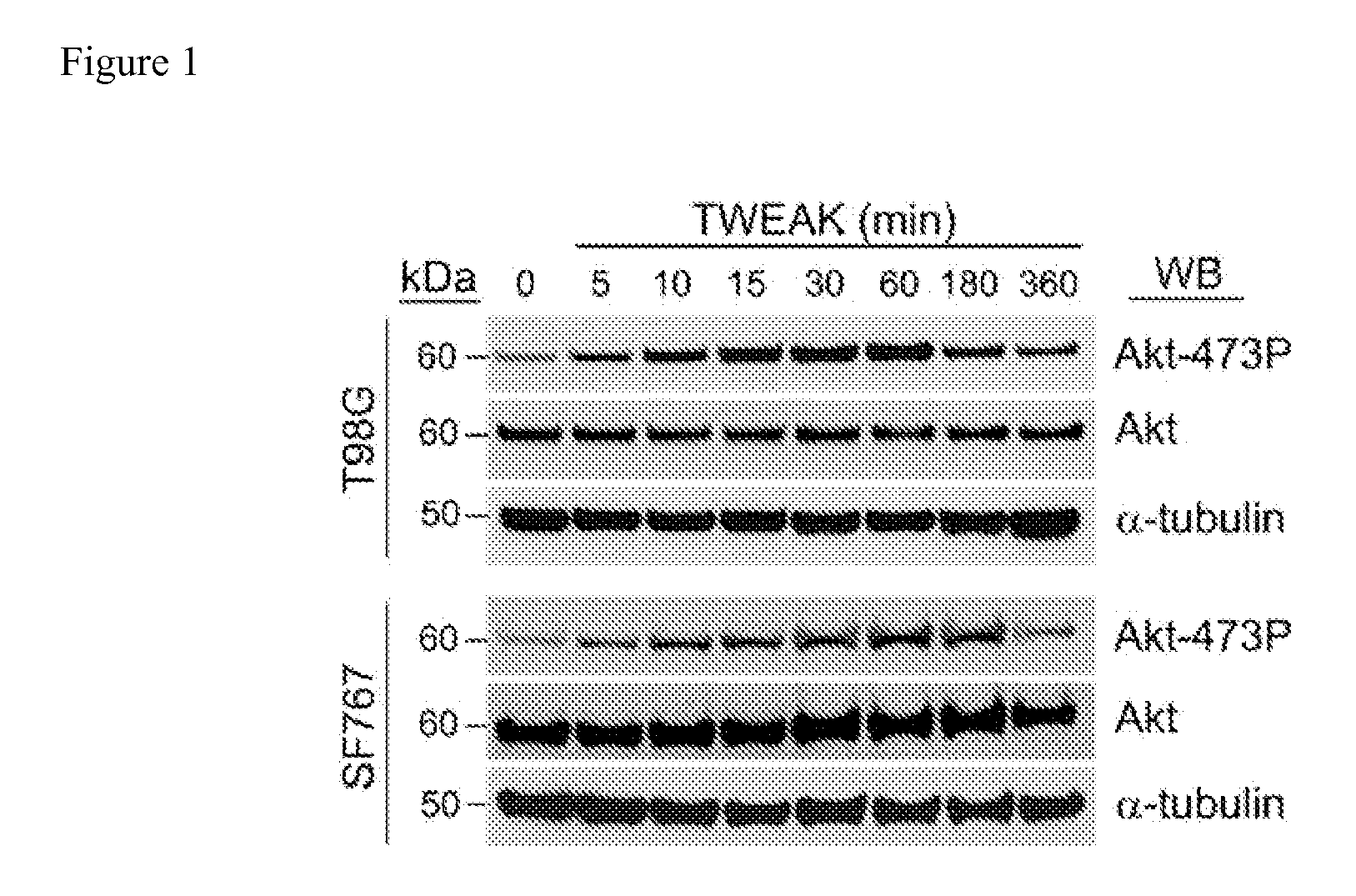
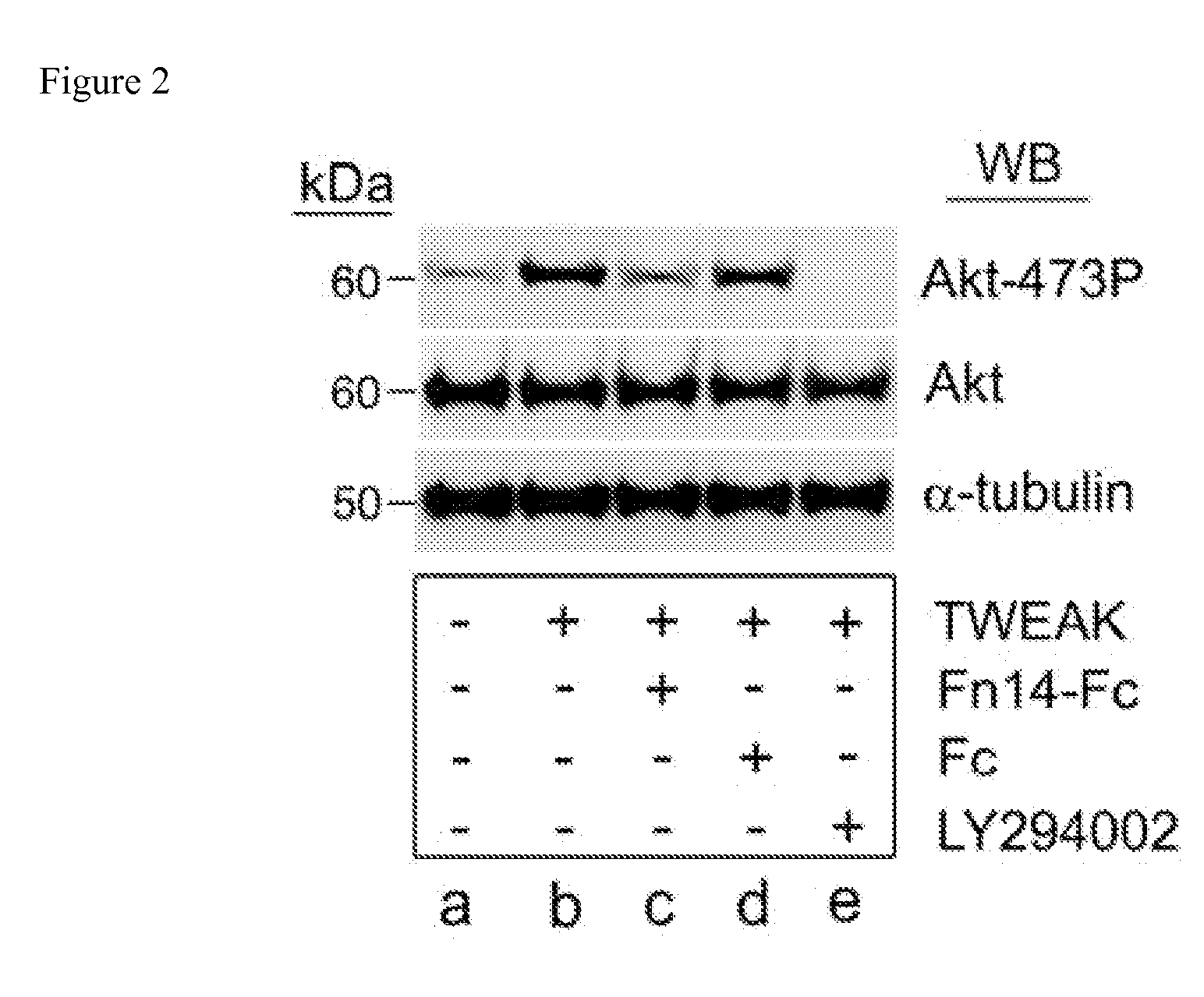
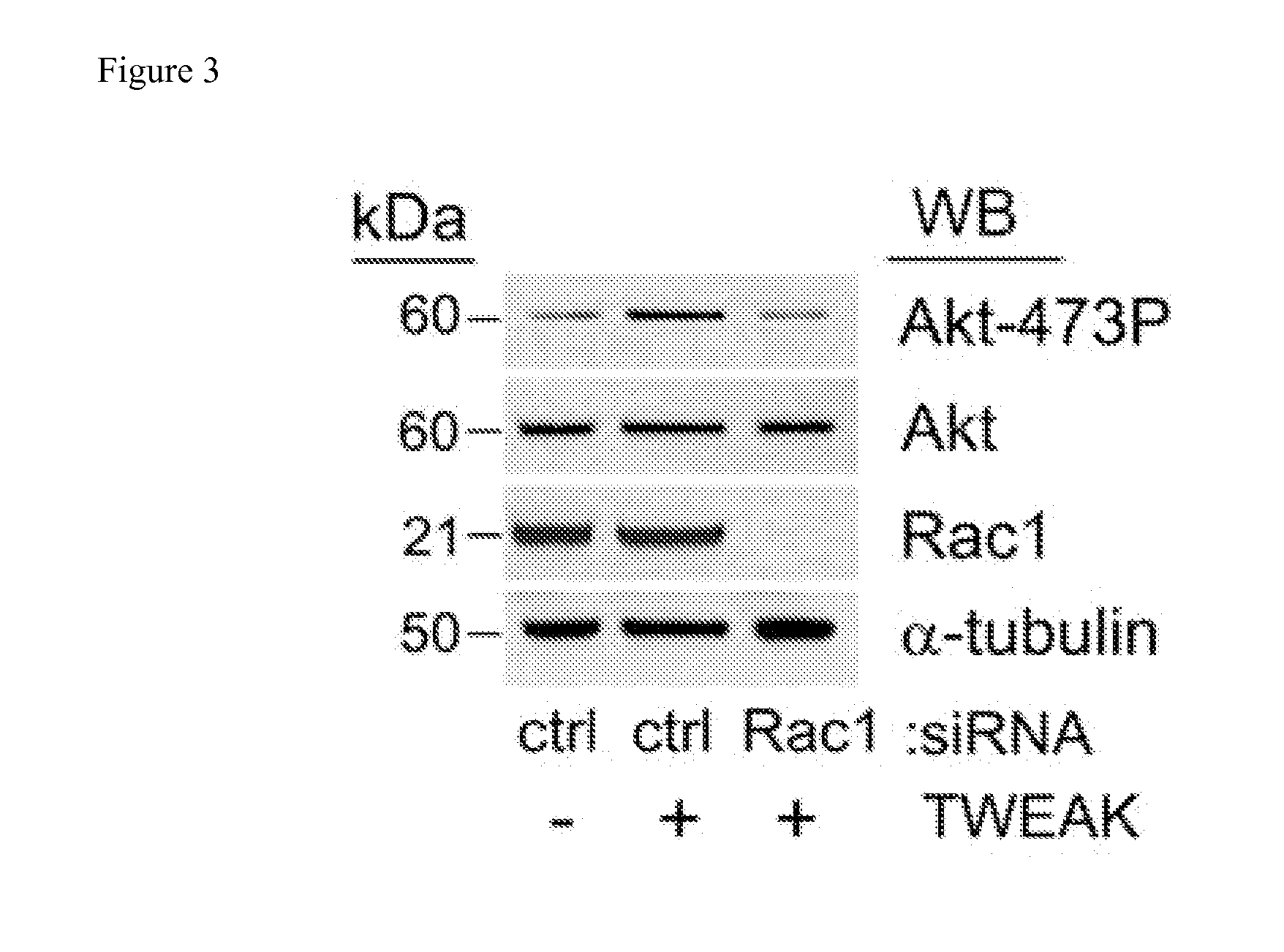
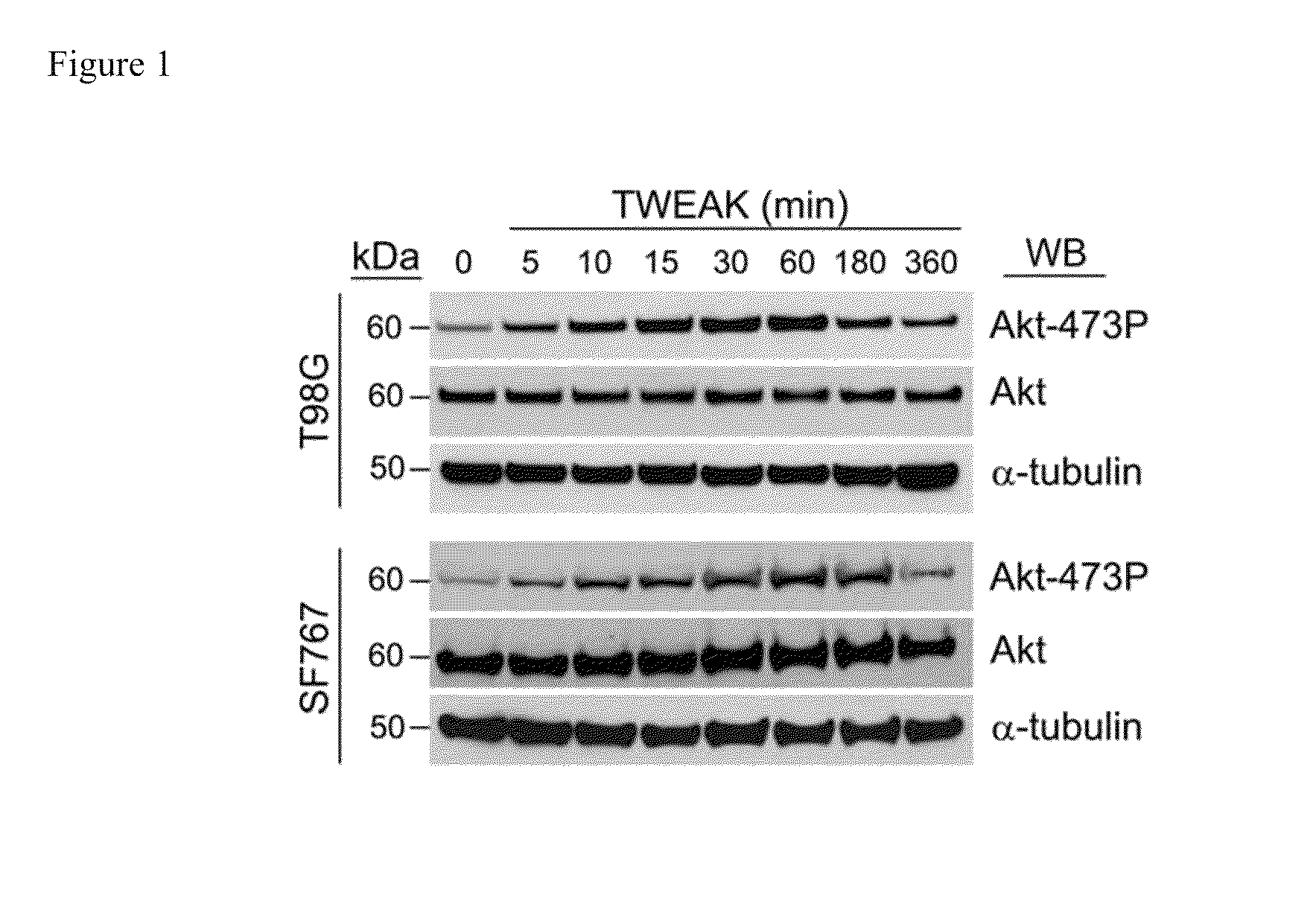
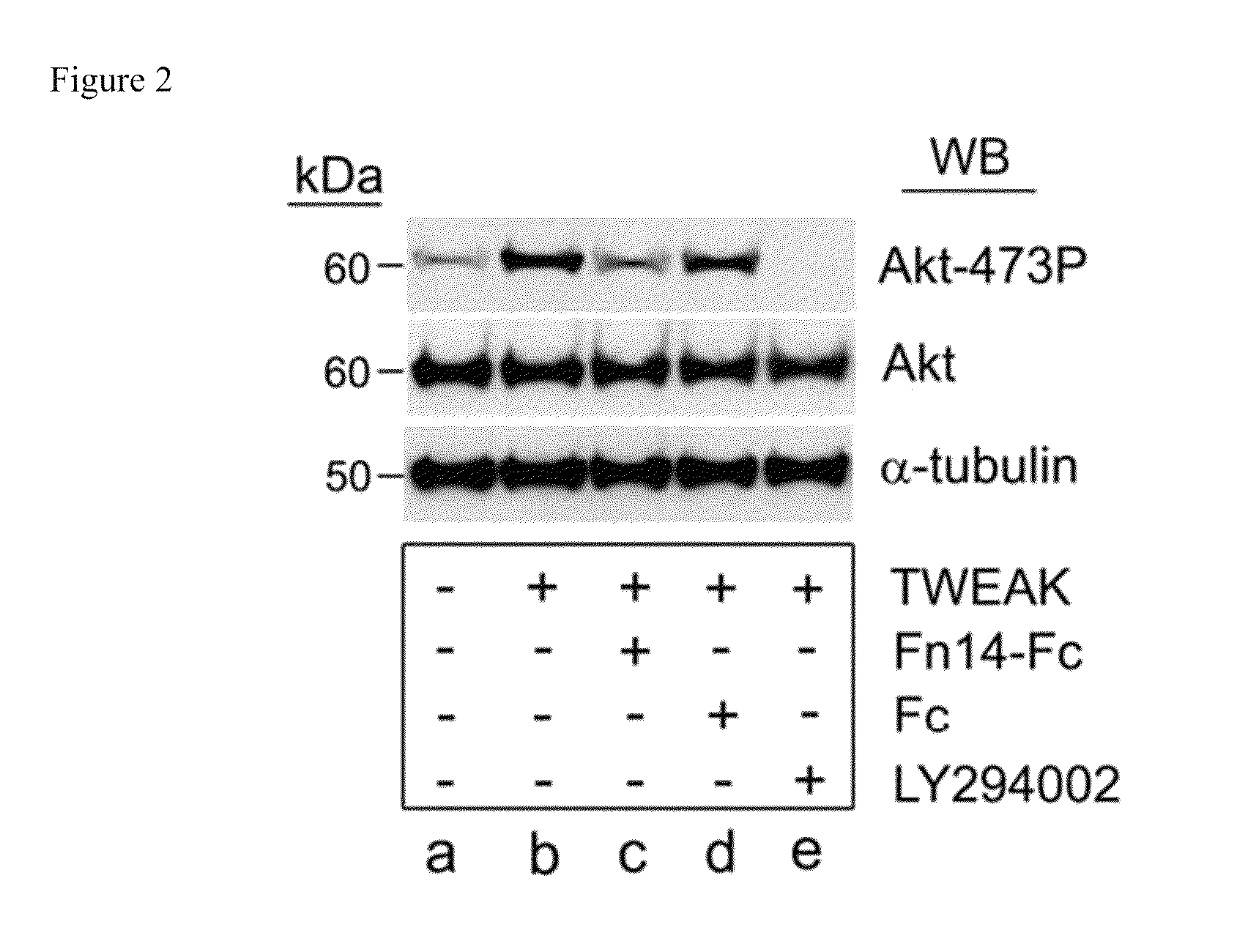
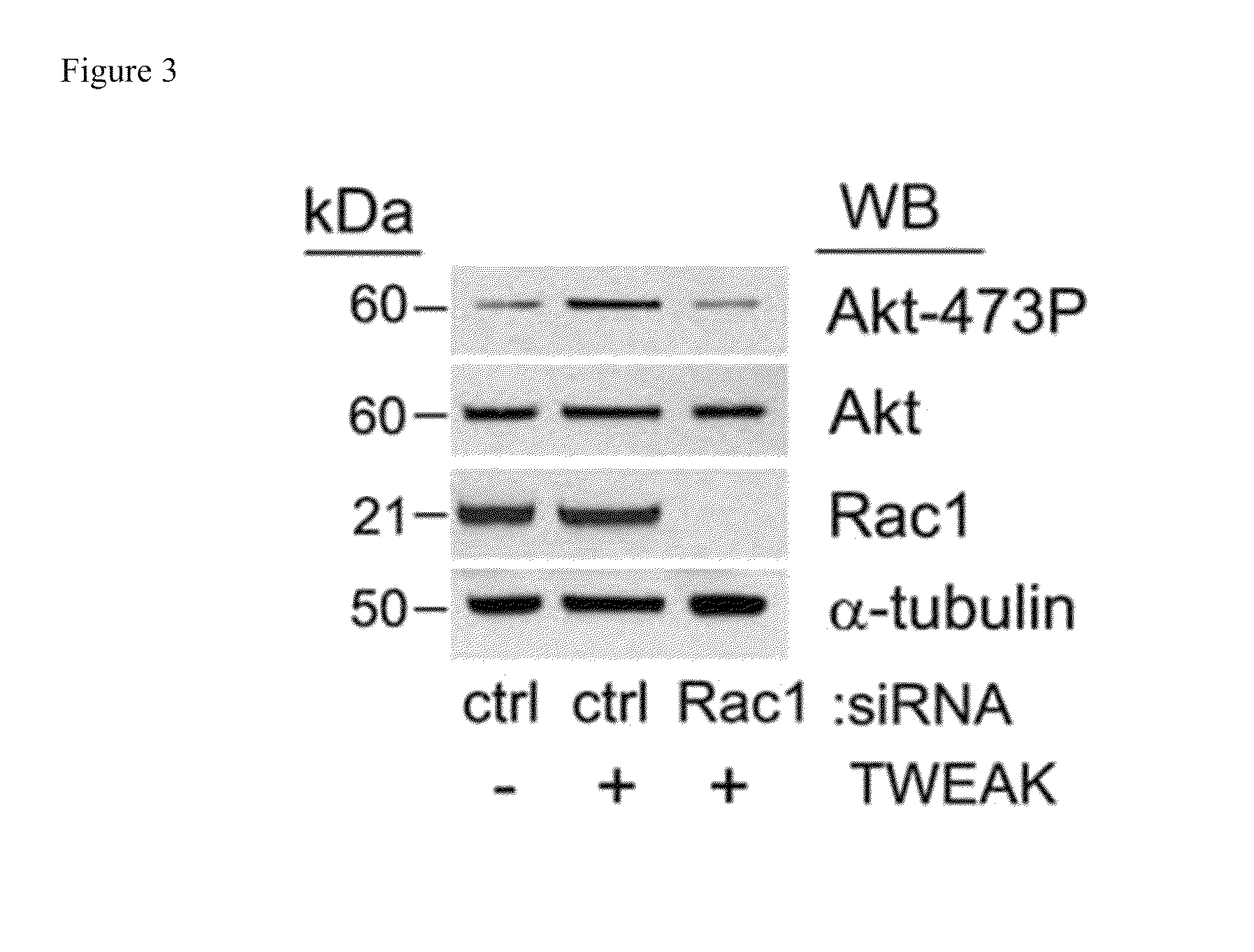
The invention encompasses methods and kits used in the identification of invasive glioblastoma based upon the expression of Akt1, Akt2, and Akt3. The methods and kits also allow prediction of disease outcome and staging of patients with regard to therapy.
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE
Systems and methods for predicting patient outcome to cancer therapy
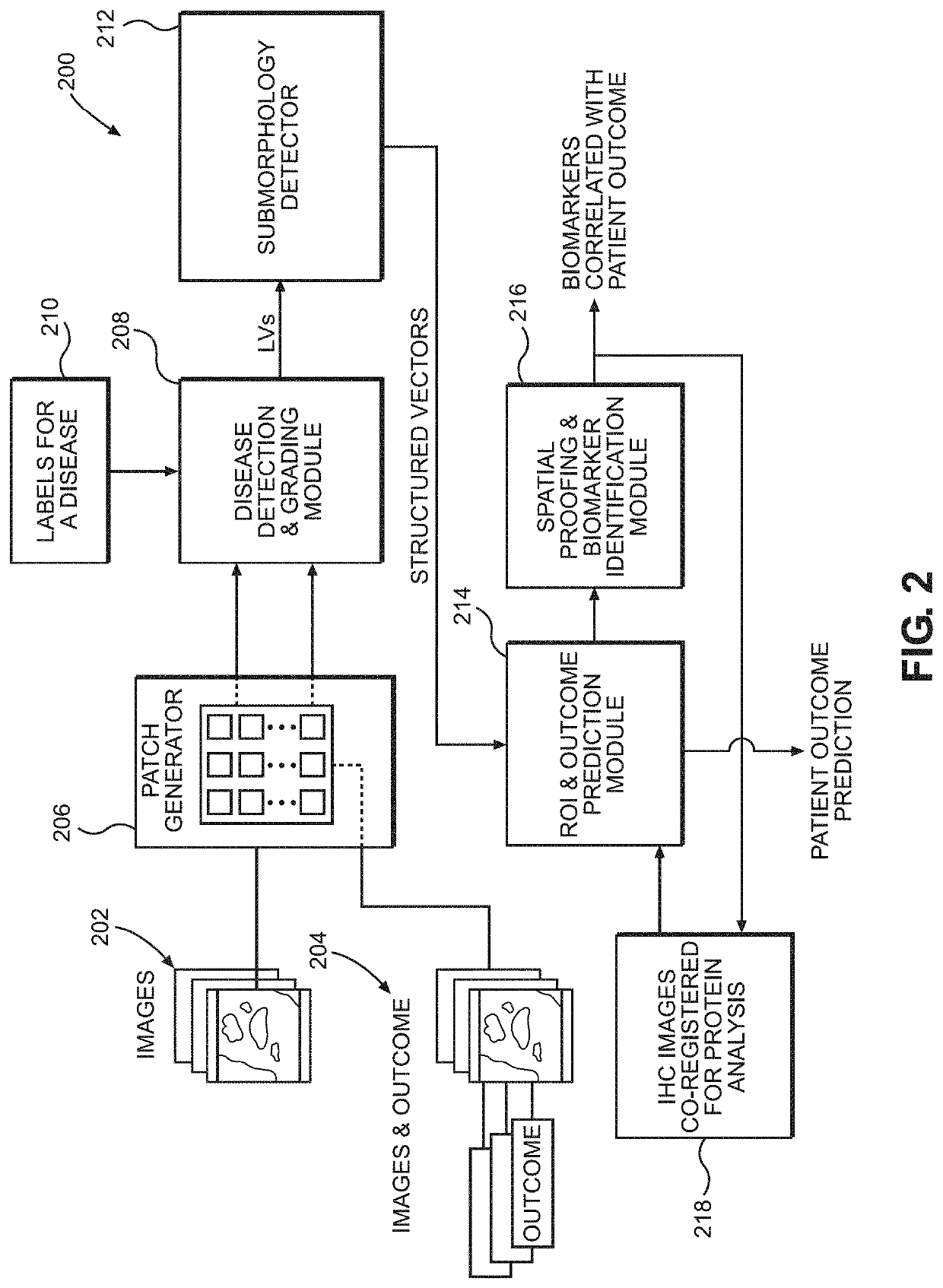
ActiveUS20210193323A1Lower confidenceMedical data miningHealth-index calculationDisease outcomeMorphological pattern
Disclosed are systems and methods for predicting patient response to a treatment option. In one embodiment, the image slides from patient tissue samples are divided into patches and morphological patterns correlated with a disease outcome are labeled and given a patch-level score, based on whether the morphological patterns occur only in patients with good outcomes or patients with poor outcomes. A patient-level score can be generated based, at least partly, on the patch-level scores. Patch-level scores can identify regions of interest for targeted biomarker identification.
Owner:PATHOMIQ INC
Molecular/genetic aberrations in surgical margins of resected pancreatic cancer represents neoplastic disease that correlates with disease outcome
InactiveUS20070031867A1Reduce unnecessary contactAggressive treatmentMicrobiological testing/measurementAbnormal tissue growthTumor target
The present invention relates to the detection of field cancerization by detection of aberrations in tumor target genes at the margins of resected tumors, and the use of such information to predict survival in cancer patients. Methods for treatment of cancer based thereon also are provided.
Owner:JOHN WAYNE CANCER INST
Comparative cancer survival models to assist physicians to choose optimal treatment
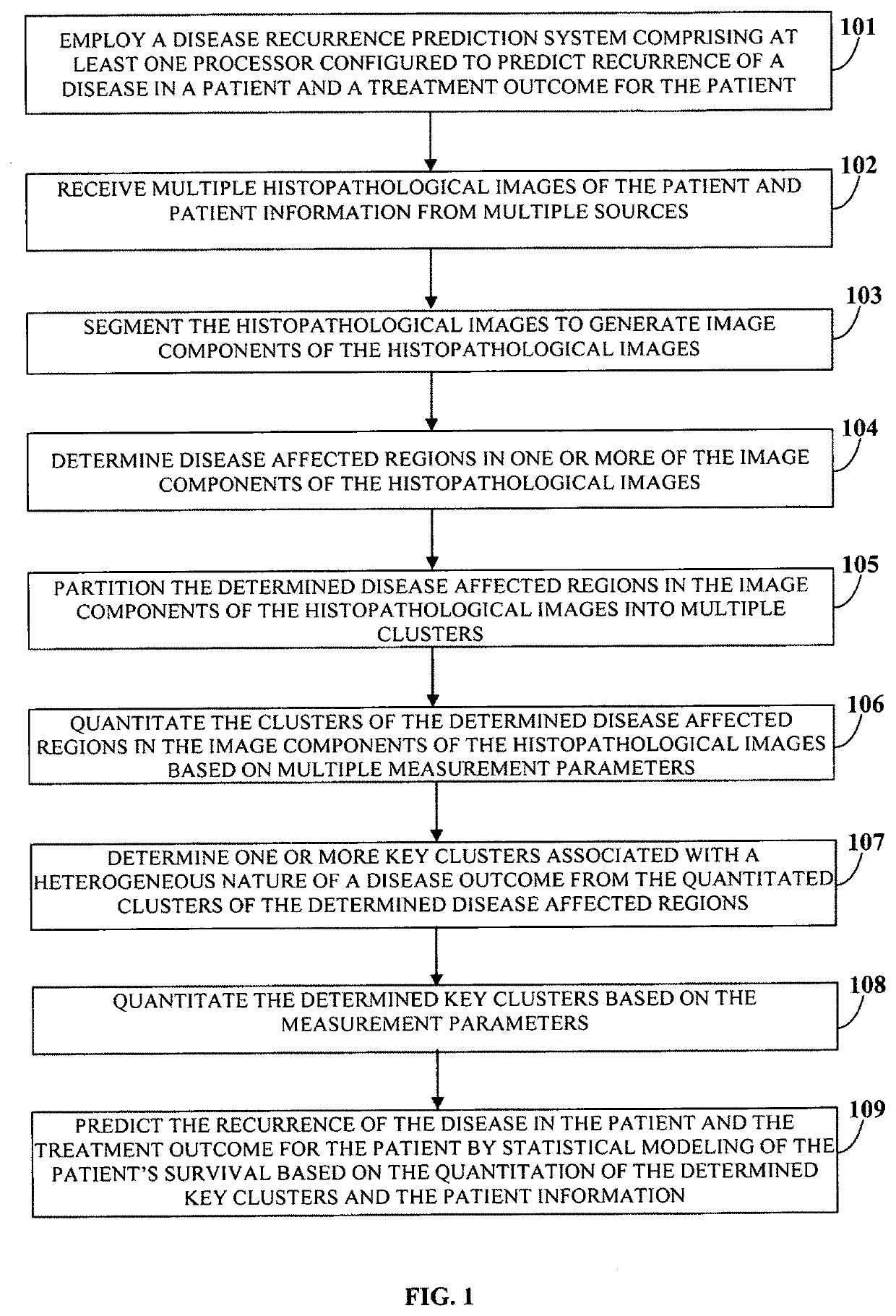
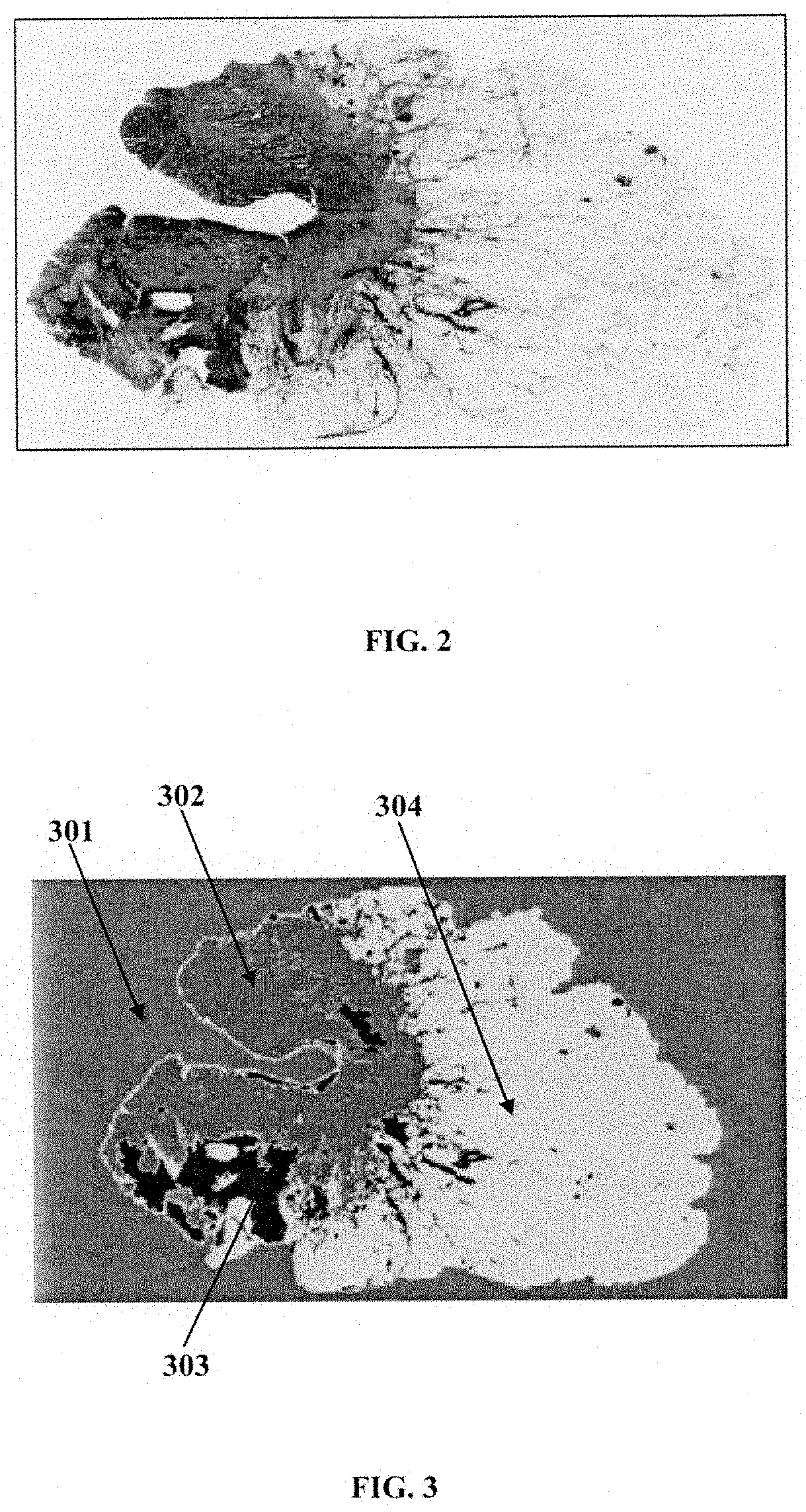
A computer implemented method and a system choosing optimal disease treatment among several possible treatment options for a patient are provided. The system computes cancer-free survival rates for each considered treatment based on predicting recurrence rate of a disease and / or cancer outcome for a particular patient. The treatment survival models use quantitative data from histopathological images of the patient, clinical data and other patient information. The system segments the histopathological images into biologically meaningful components; automatically determines disease-affected regions in one or more of the segmented image components. The system also partitions the disease-affected regions in each image into a number clusters. Those that are determined to be the most associated with the disease outcome are used as a source of the imaging information for the survival modeling. Optimal treatment is suggested as the treatment with probability of the cancer free survival within a certain time period is maximized.
Owner:TEVEROVSKIY MIKHAIL
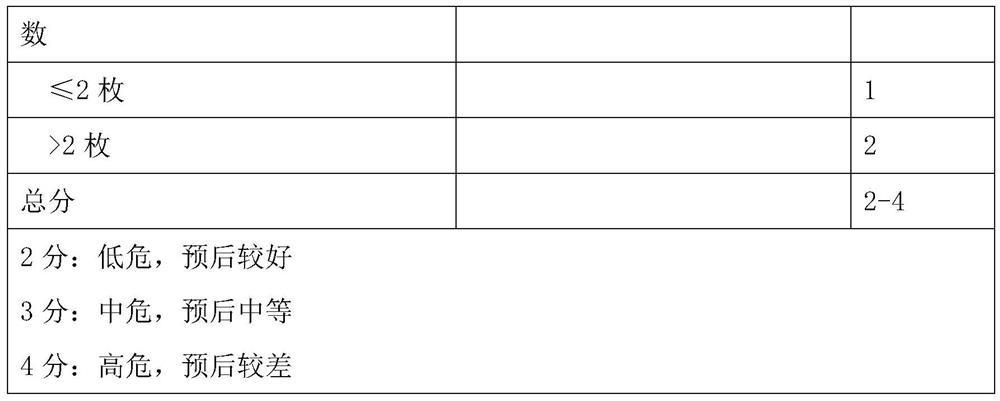
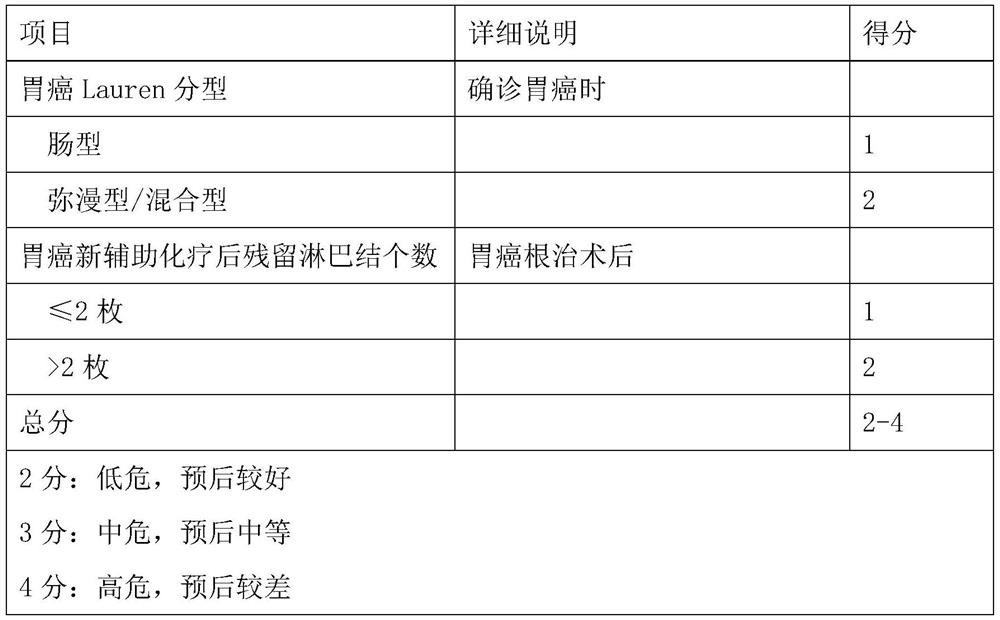
Model for evaluating gastric cancer prognosis based on Lauren typing and postoperative residual lymph nodes and application
PendingCN112837815AEfficient communicationEffective predictionMechanical/radiation/invasive therapiesHealth-index calculationPhysician patient communicationOncology
The invention relates to a model for evaluating gastric cancer prognosis based on Lauren typing and postoperative residual lymph nodes and application, and belongs to the technical field of biomedical detection. According to the invention, the number of residual lymph nodes after Lauren typing and neoadjuvant chemotherapy is comprehensively used as a prognosis evaluation index after neoadjuvant chemotherapy of gastric cancer, and a prognosis evaluation model for patients after neoadjuvant chemotherapy of gastric cancer is established. Through clinical verification, the evaluation model can effectively pre-judge disease outcome after neoadjuvant chemotherapy of gastric cancer, and is more accurate than the traditional Japan JGCA standard and German Becker standard. The invention provides reference information for prognosis of tumor patients, so that clinical doctors are helped to formulate the most reasonable diagnosis and treatment plan for the tumor patients, and timely and effective doctor-patient communication is facilitated.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Use of monoclonal antibodies to distinguish protein conformational isoforms
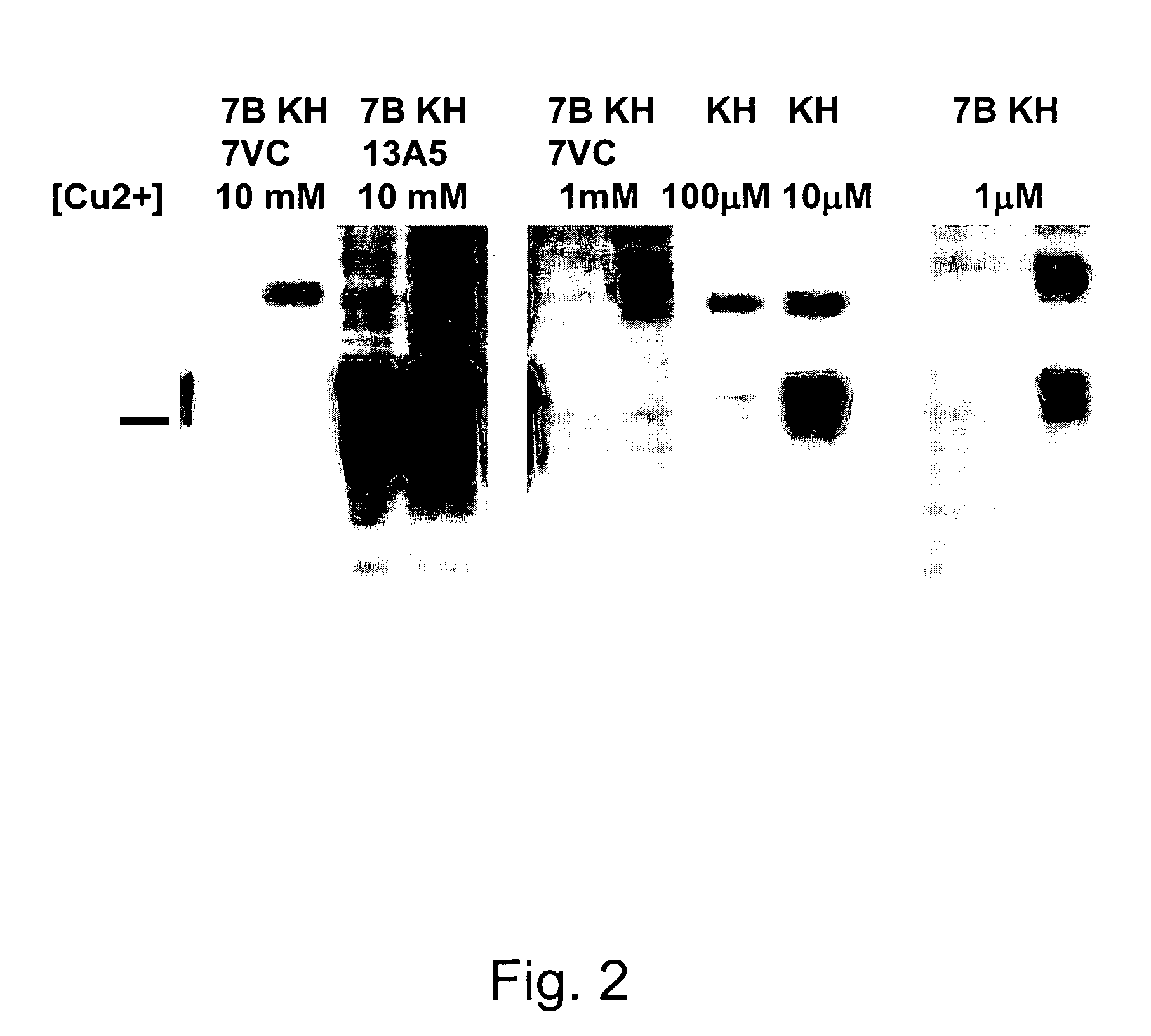
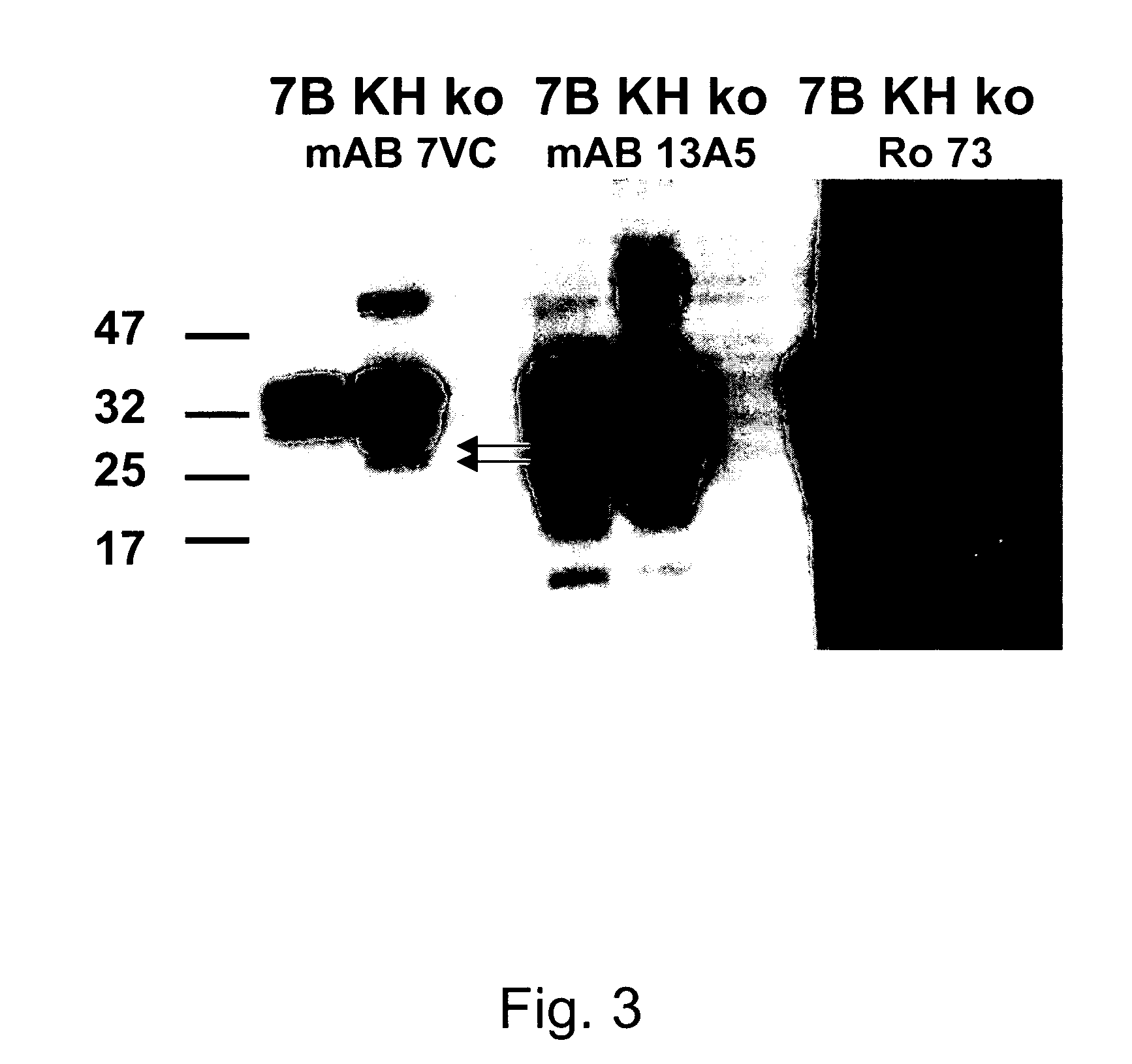
InactiveUS20070281318A1Promote differentiationInhibit PrP expressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsScreening techniquesActive immunization
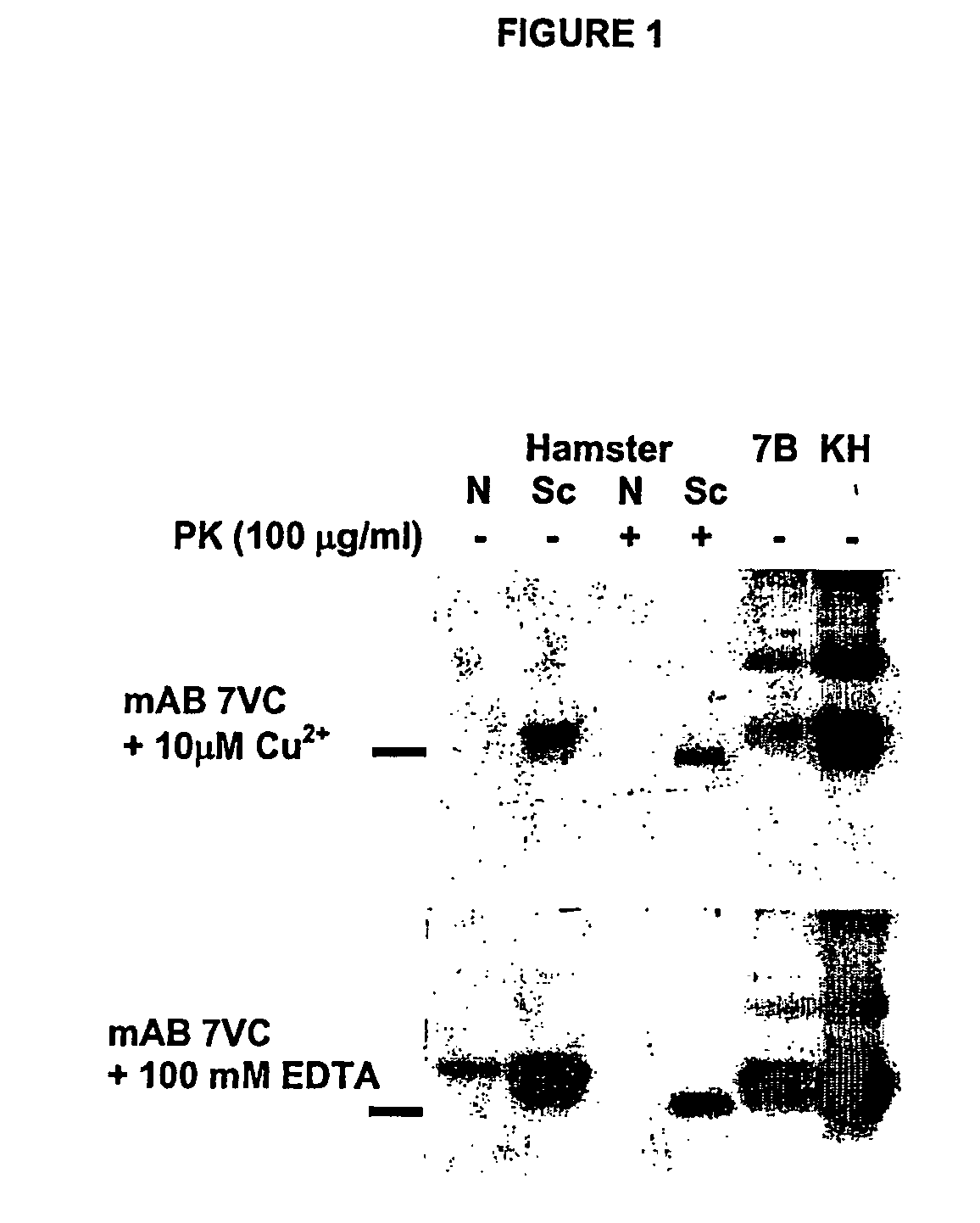
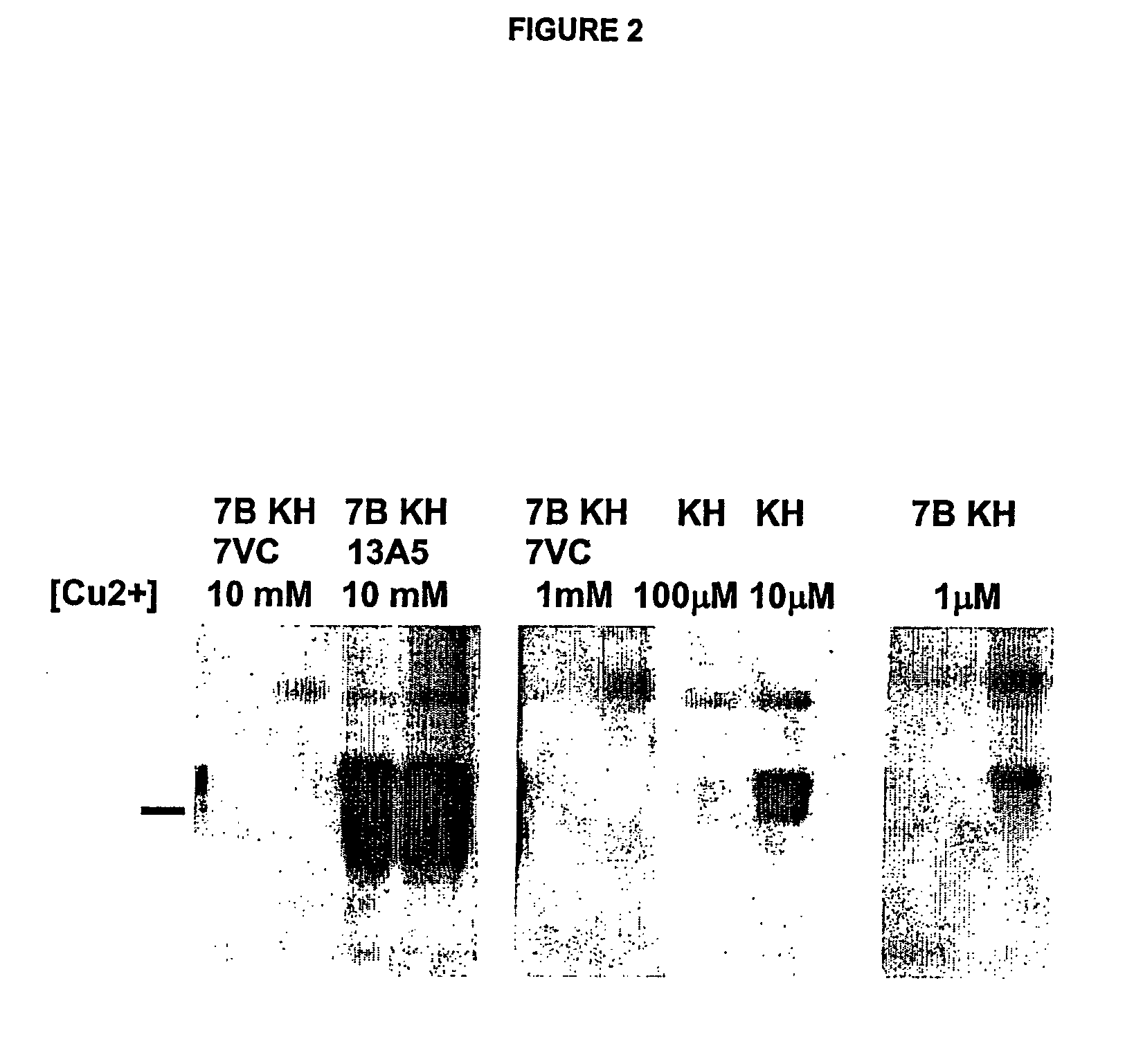
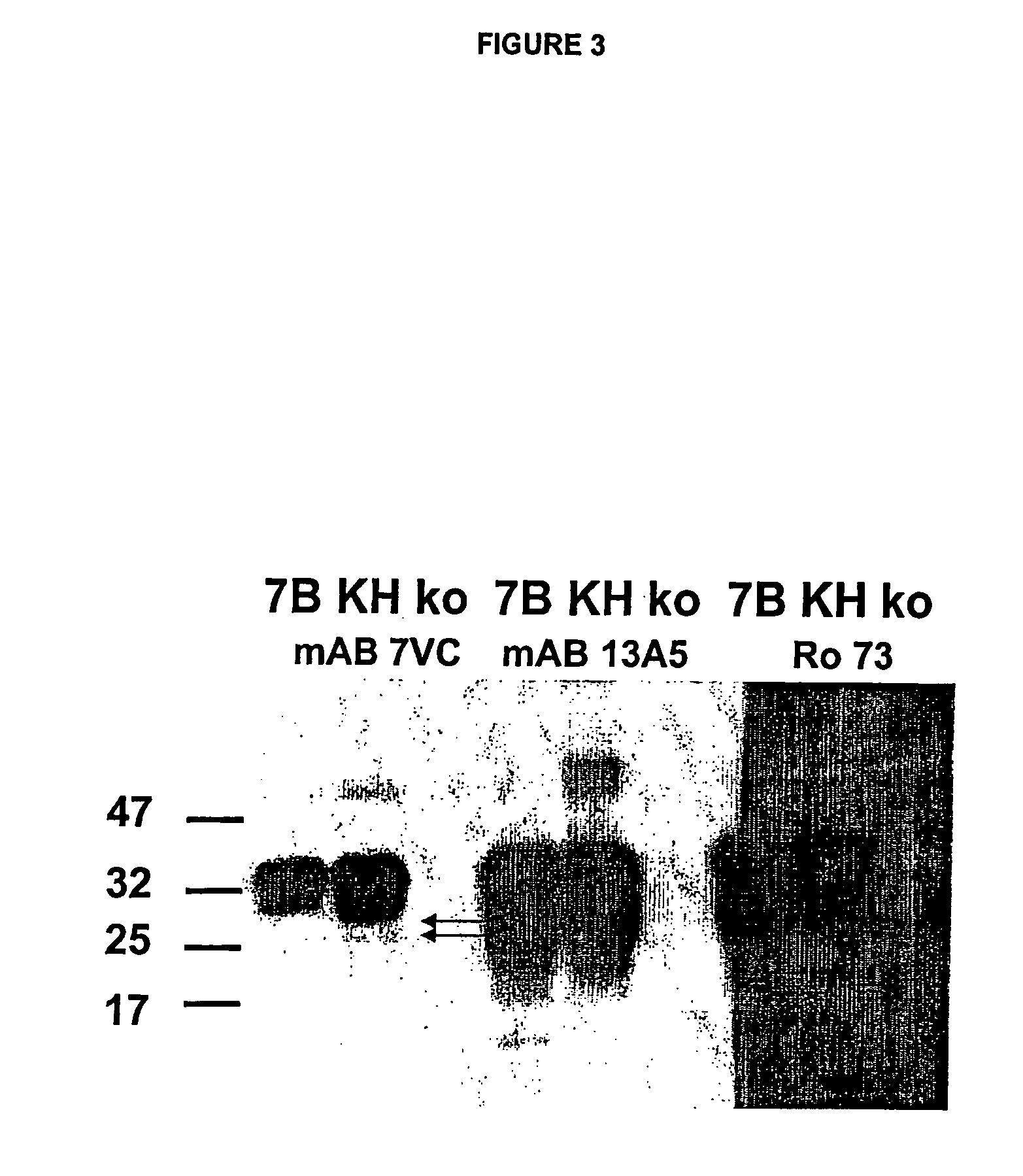
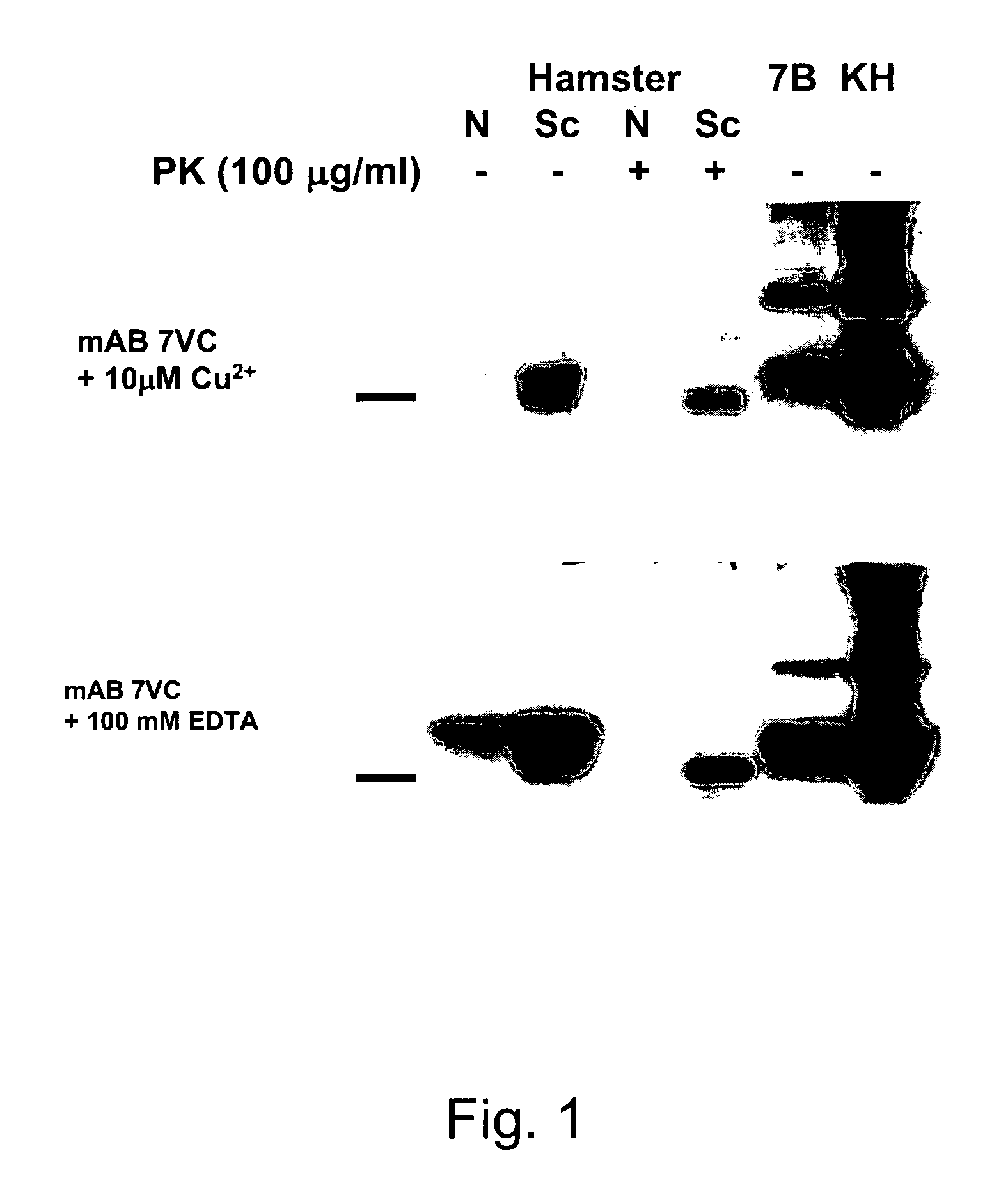
Methods of preparing monoclonal antibodies that differentially bind to a single conformer of a protein of interest are described. Passive immunization using these antibodies as well as use of conformer-specific antibodies as diagnostic reagents for the purpose of stratification of patient populations with regards to disease outcome, drug efficacy or drug sensitivity is also disclosed as well as active immunization with the protein conformer. In the screening techniques, detection can be for example by tissue immunostaining, western blotting or solution IP. A specific mab termed 7VC which shows conformation specificity to CtmPrP, a prion protein conformer that triggers neurodegeneration under specific assay conditions of pH and copper concentration, is described. A second specific antibody termed 19B10 shows conformation specificity for NtmPrP, a prion protein conformer that downregulates total PrP expression and effects cell differentiation.
Owner:HEINRICH HEINE UNIV OF DUSSELDORF +1
Method for breast cancer recurrence prediction under endocrine treatment
ActiveUS20170067118A1Reliable predictionMicrobiological testing/measurementMaterial analysisDisease outcomeEndocrine therapy
The present invention relates to methods, kits and systems for the prognosis of the disease outcome of breast cancer, said method comprising:(a) determining in a tumor sample from said patient the RNA expression levels of at least 2 of the following 9 genes: UBE2C, BIRC5, RACGAP1, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP(b) mathematically combining expression level values for the genes of the said set which values were determined in the tumor sample to yield a combined score, wherein said combined score is indicative of a prognosis of said patient; and kits and systems for performing said method.
Owner:SIVIDON DIAGNOSTICS
Plasma metabolization micromolecule marker related to human non-small-cell lung cancer and application of plasma metabolization micromolecule marker
InactiveCN105203683ARepair inhibitionIncrease contractilityComponent separationMetaboliteConfidence interval
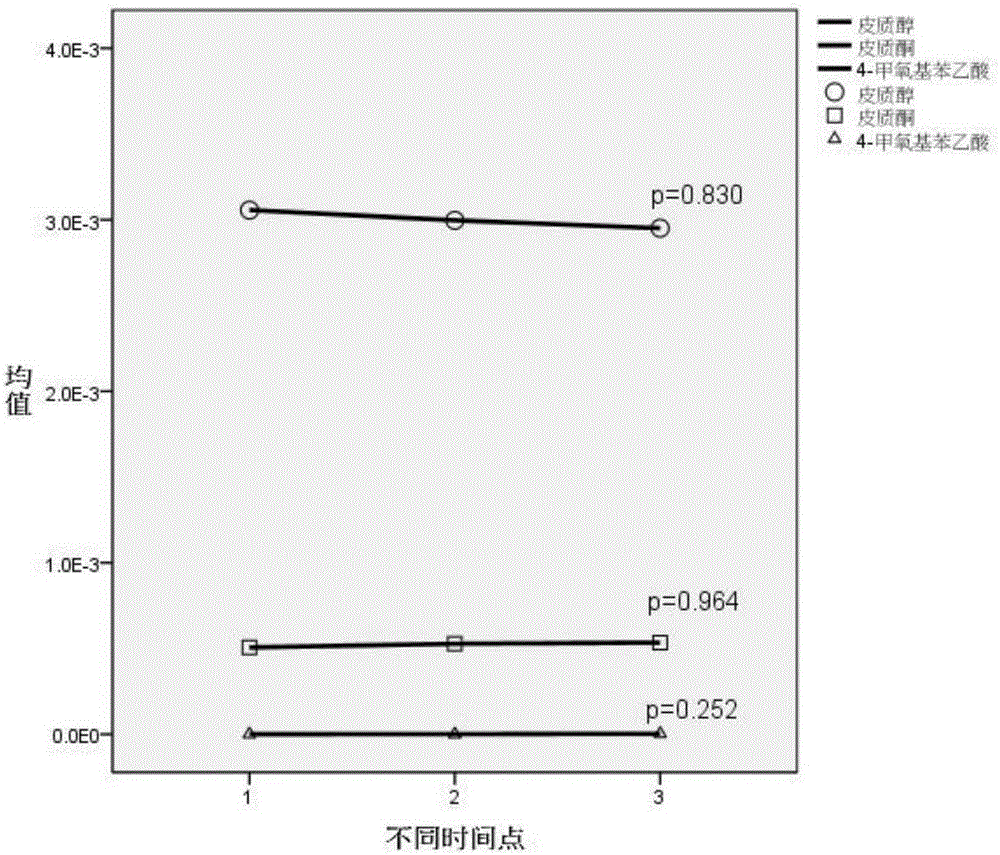
The invention belongs to the field of analytical chemistry and clinical medicine and relates to a plasma metabolization micromolecule marker related to the human non-small-cell lung cancer and an application of the plasma metabolization micromolecule marker. The plasma metabolization micromolecule marker related to the human non-small-cell lung cancer is one or more of cortisol, corticosterone and 4-methoxyphenylacetic acid. The plasma metabolization micromolecule marker is prepared from cortisol, corticosterone and 4-methoxyphenylacetic acid. The content range (95% confidence interval) of cortisol is 0.00018-0.00067, the content range (95% confidence interval) of corticosterone is 0.000029-0.00010, the content range (95% confidence interval) of 4-methoxyphenylacetic acid is 0.000015-0.000022, and metabolite can prompt occurrence of tumors within the ranges. The horizontal range, corresponding to a normal group, of cortisol is 0.0030-0.0037, the horizontal range, corresponding to the normal group, of corticosterone is 0.00044-0.00056, and the horizontal range, corresponding to the normal group, of 4-methoxyphenylacetic acid is 7.39 E-07-2.09 E-06. The plasma metabolization micromolecule marker is a novel biomarker, compared with a traditional protein biomarker, the relevance between the marker and the disease outcome is higher, and the plasma metabolization micromolecule marker is stable, minimally invasive, easy to detect and accurate in quantitation.
Owner:JIANGSU PROVINCE HOSPITAL
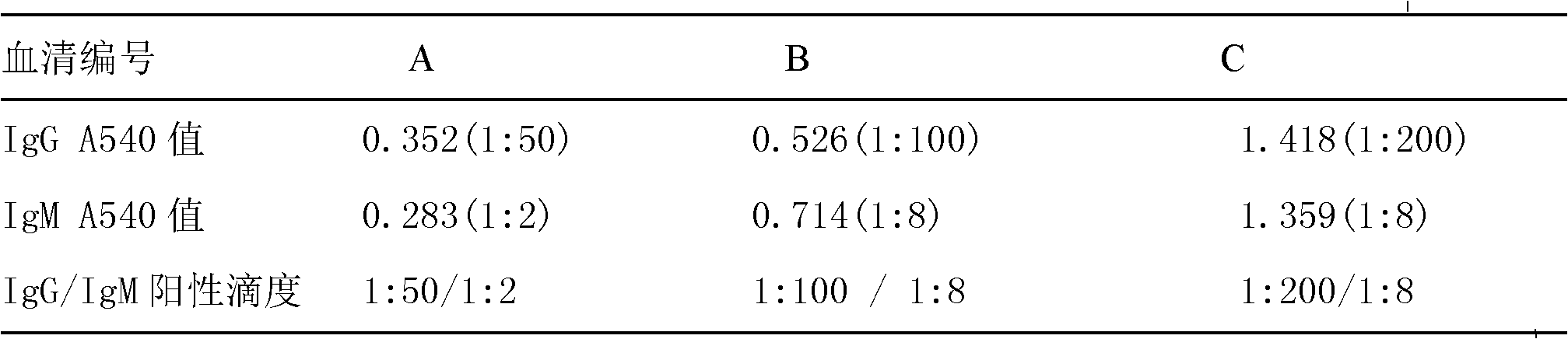
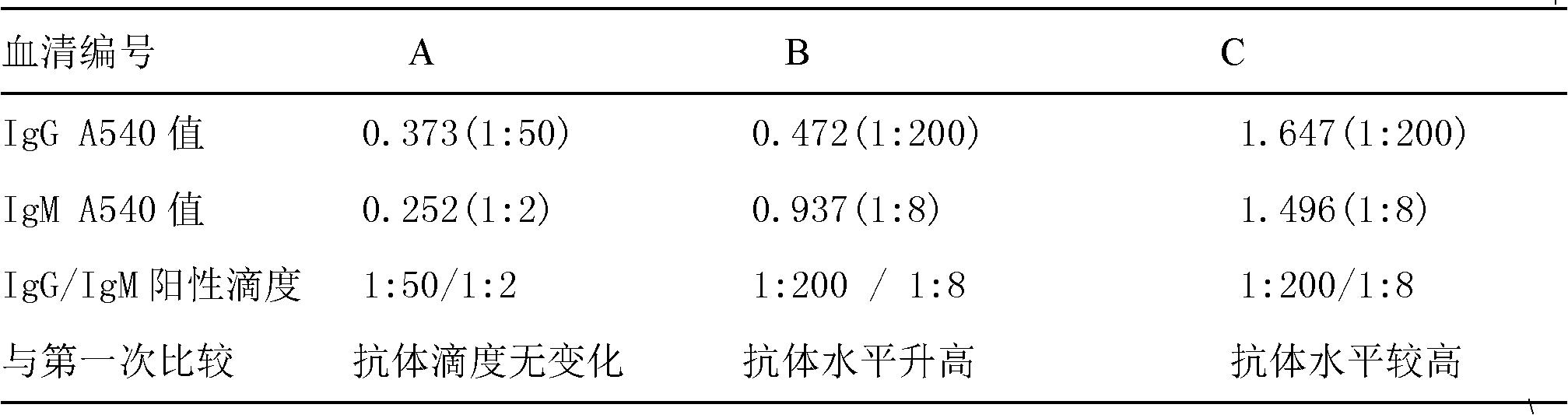
Application of suite reagent for detecting EV71-VP1 antibody of hand-foot-mouth disease patient in disease outcome prediction
InactiveCN101936994APredictable clinical severityClinical severe disease can be preventedMaterial analysisDisease outcomeSorbent
The invention discloses a suite reagent for titrating the titer of an EV71IgM antibody and an EV71IgG antibody of a hand-foot-mouth disease patient, comprising an ELISA (Enzyme Linked Immuno Sorbent Assay) plate and a detection reagent. The ELISA plate of the EV71IgM antibody comprises a polystyrene board, 1gM capture antibody is coated in polystyrene board holes, the ELISA plate of the EV71IgG antibody comprises a polystyrene board, and 1gM capture antibody is coated in the polystyrene board holes, and the detection reagent is EV71VP1 labeled by HRP (Horseradish Peroxidase). The suite reagent of the invention can be used for detecting the antibody titer of the hand-foot-mouth disease patient, the detection result can be used as a judgment result for judging disease outcome so that a clinical severe case can be predicted, prevented and controlled to lessen the probability of death cases.
Owner:INST OF BASIC MEDICINE OF SAMS +1
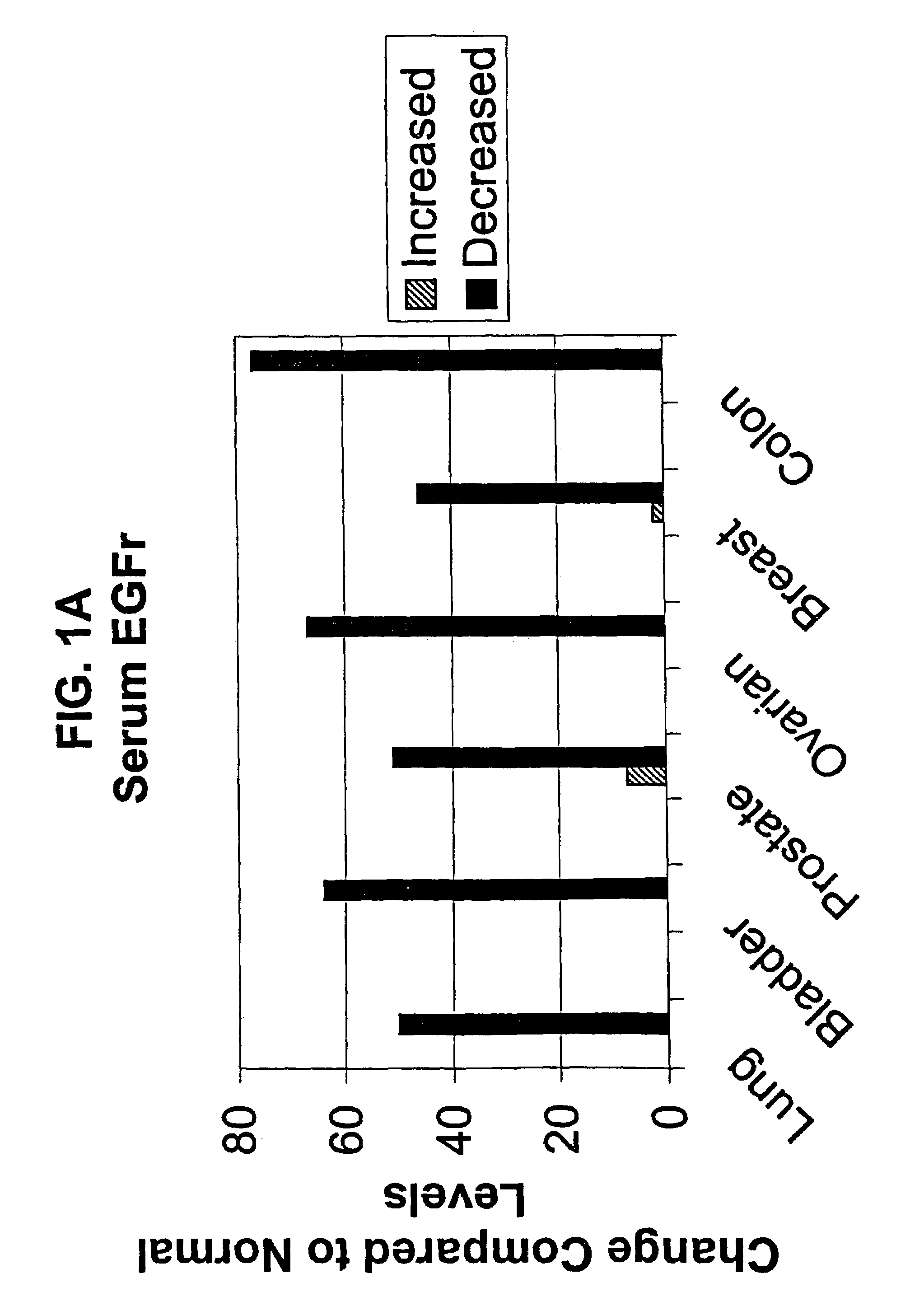
Assays for cancer patient monitoring based on levels of epidermal growth factor receptor (EGFR) extracellular domain (ECD) analyte, alone or in combination with other analytes, in body fluid samples
InactiveUS7473534B2Reduce the burden onReduce removalMicrobiological testing/measurementDisease diagnosisHer DiseaseDisease progression
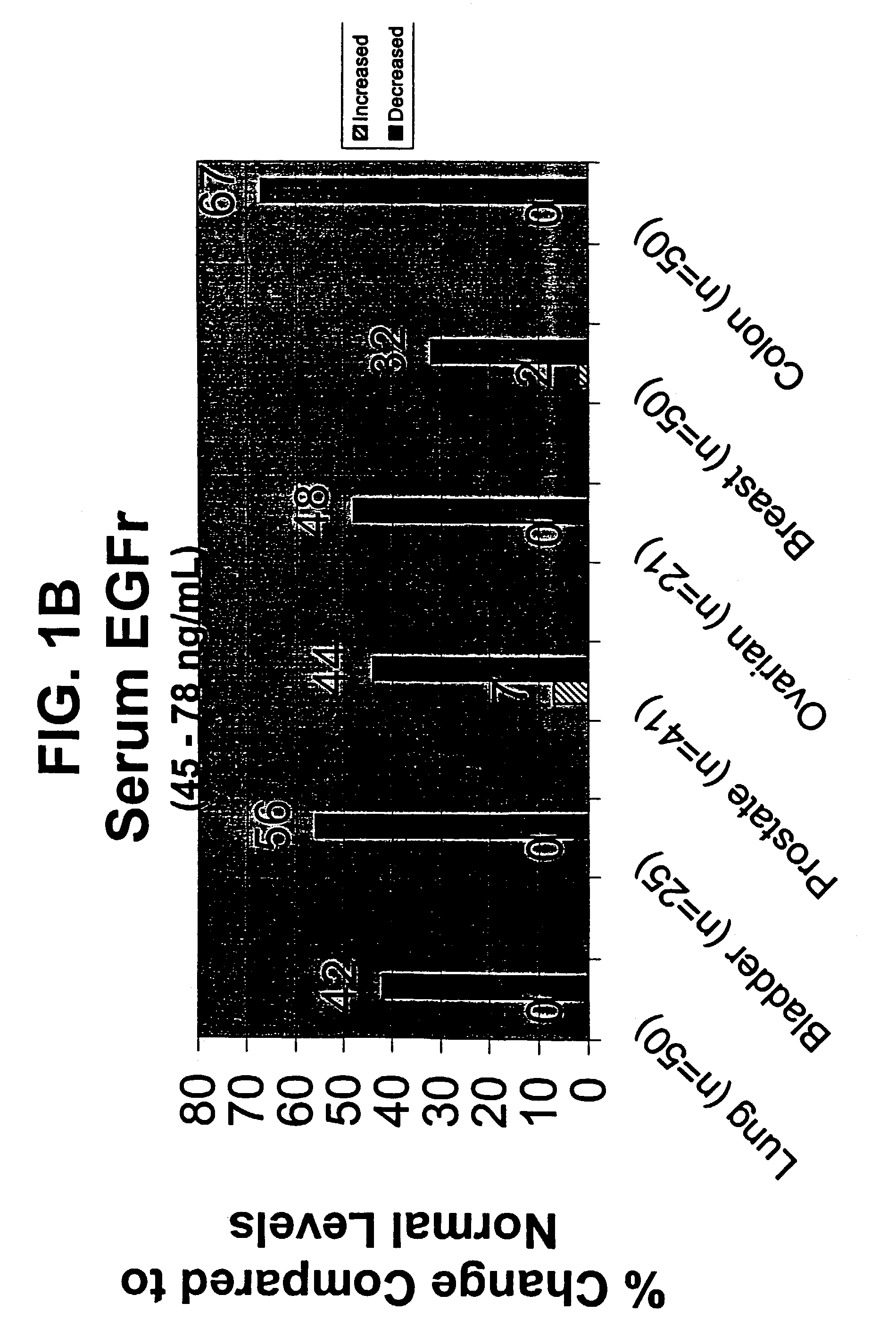
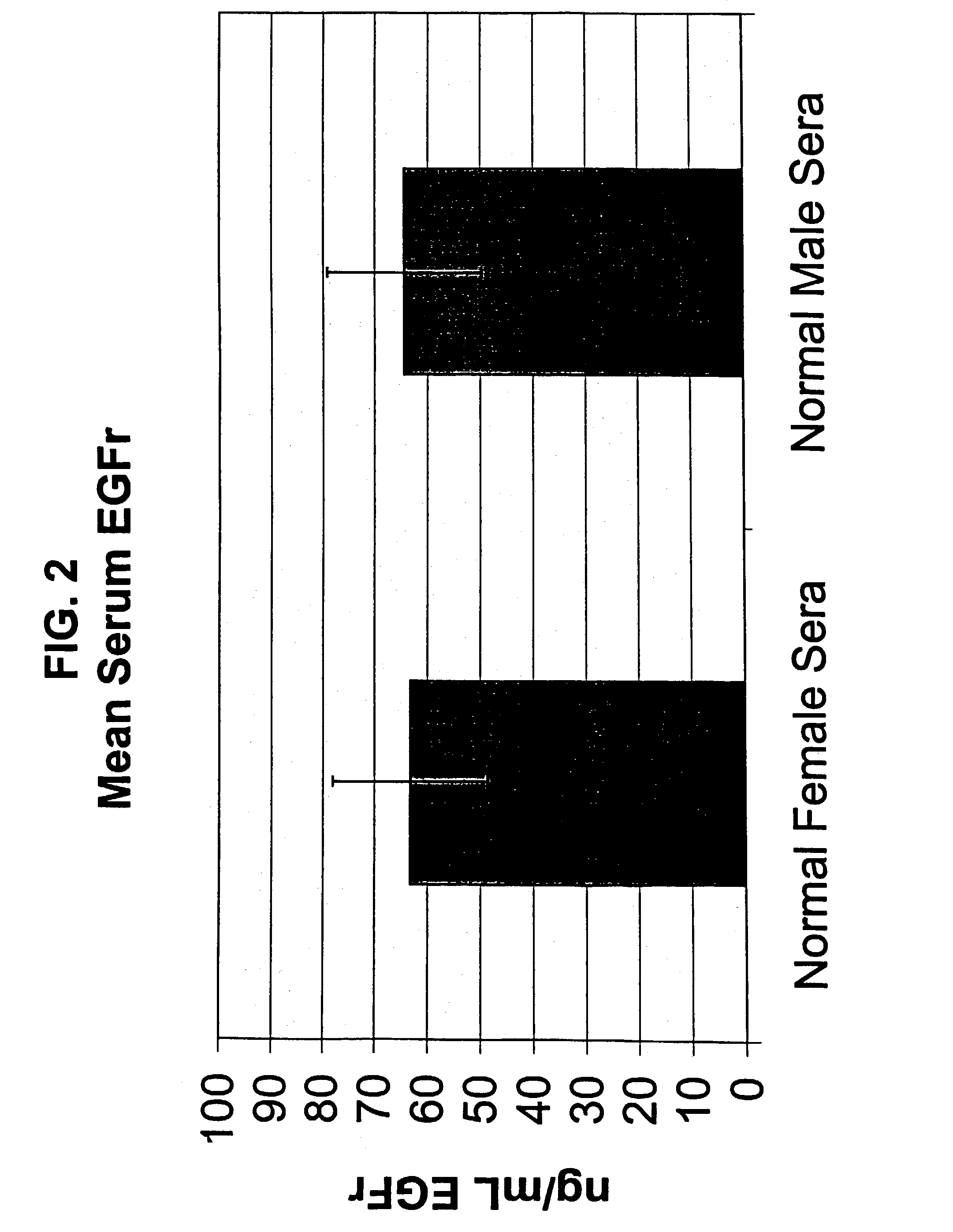
The present invention describes clinically and medically important methods of monitoring the outcome of a cancer patient who is suffering from disease or who is undergoing treatment or therapy for his or her disease. More specifically, the invention provides a method of monitoring the progression of disease or cancer treatment effectiveness in a cancer patient by measuring the level of the extracellular domain (ECD) of the epidermal growth factor receptor (EGFR) in a sample taken from the cancer patient, preferably, before treatment, at the start of treatment, and at various time intervals during treatment, wherein a decrease in the level of the ECD of the EGFR in the cancer patient compared with the level of the ECD of the EGFR in normal control individuals serves as an indicator of cancer advancement or progression and / or a lack of treatment effectiveness for the patient. As another aspect of determining disease outcome and survival, the invention further provides assessing both EGFR and HER-2 / neu levels, in combination, in a patient sample. A finding of decreased levels of EGFR concomitantly with elevated or increased levels of HER-2 / neu relative to control levels indicates poor outcome and short time to progression.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Compound long-acting injection containing enrofloxacin and meloxicam, and preparation method of compound long-acting injection
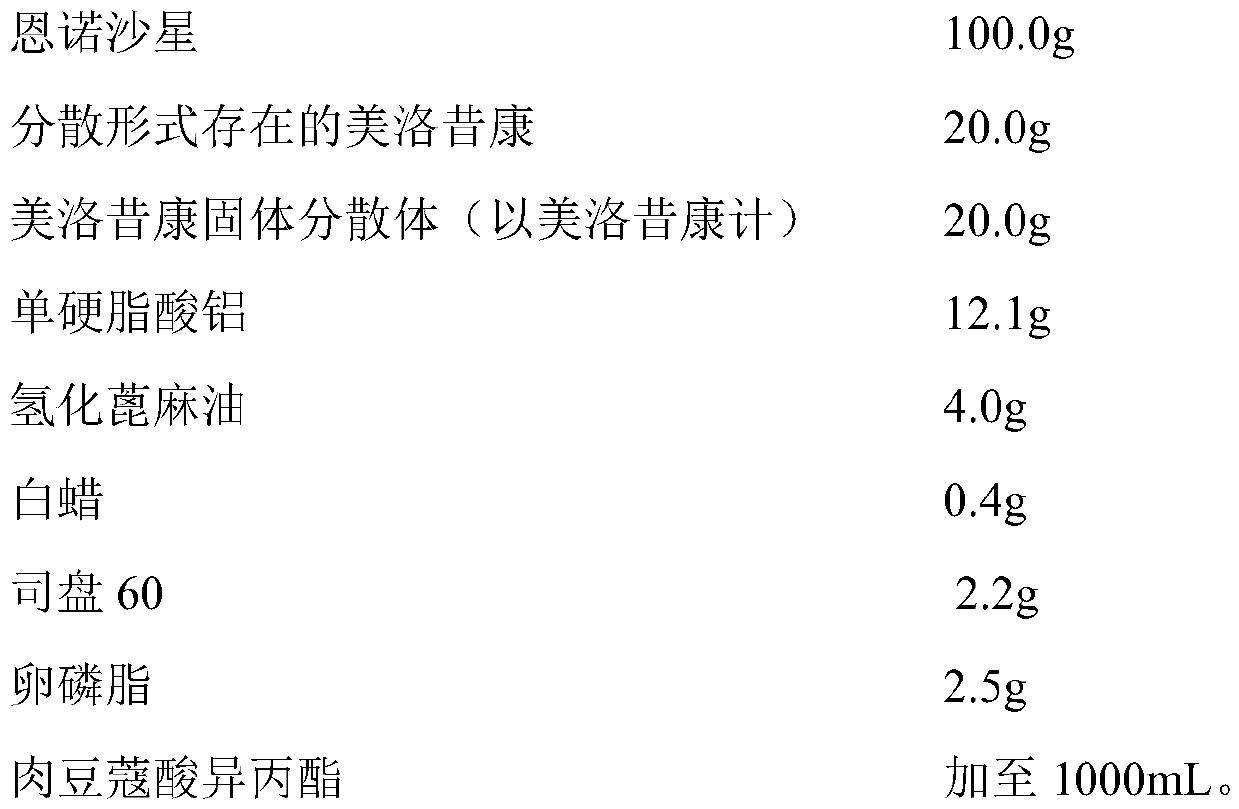
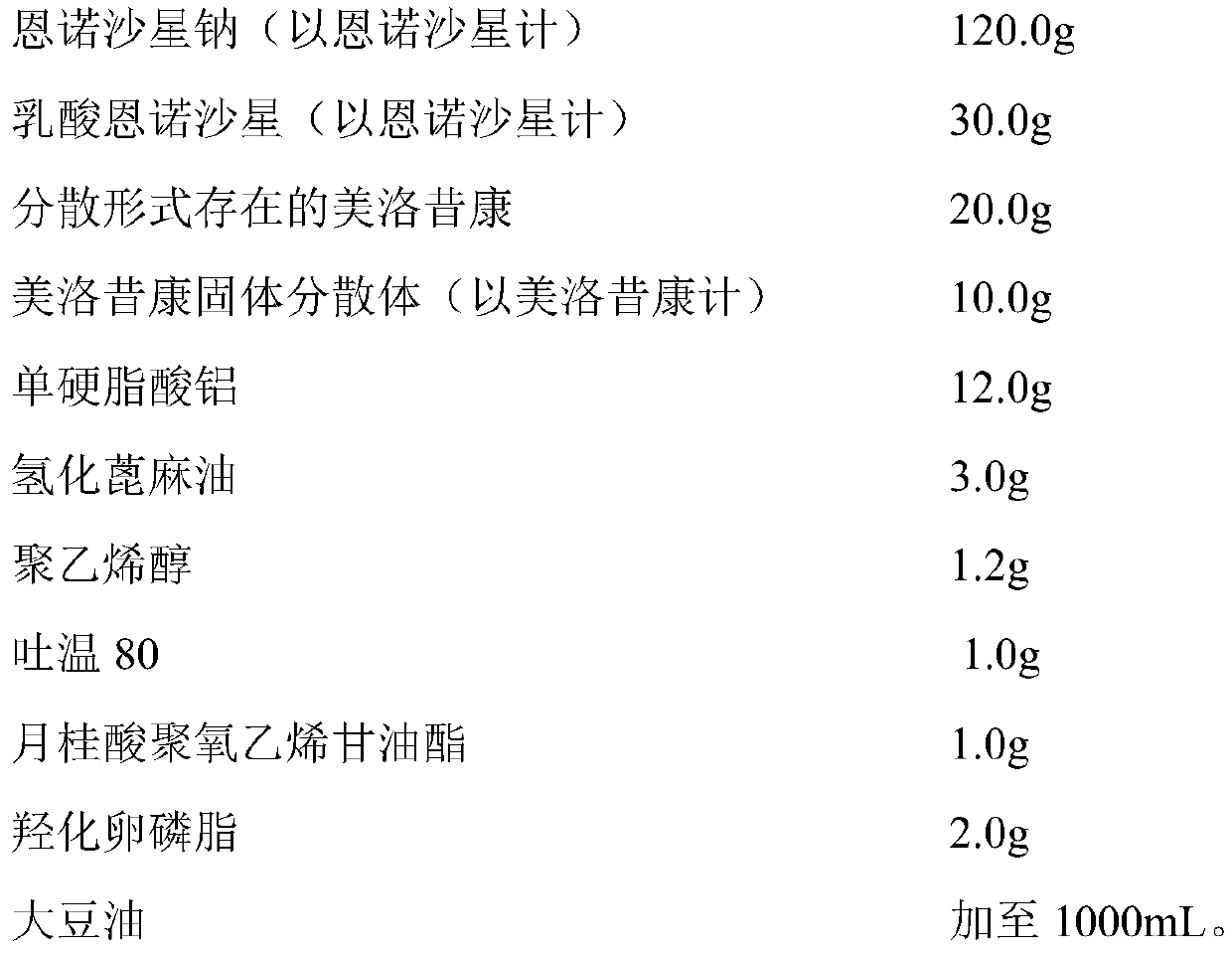
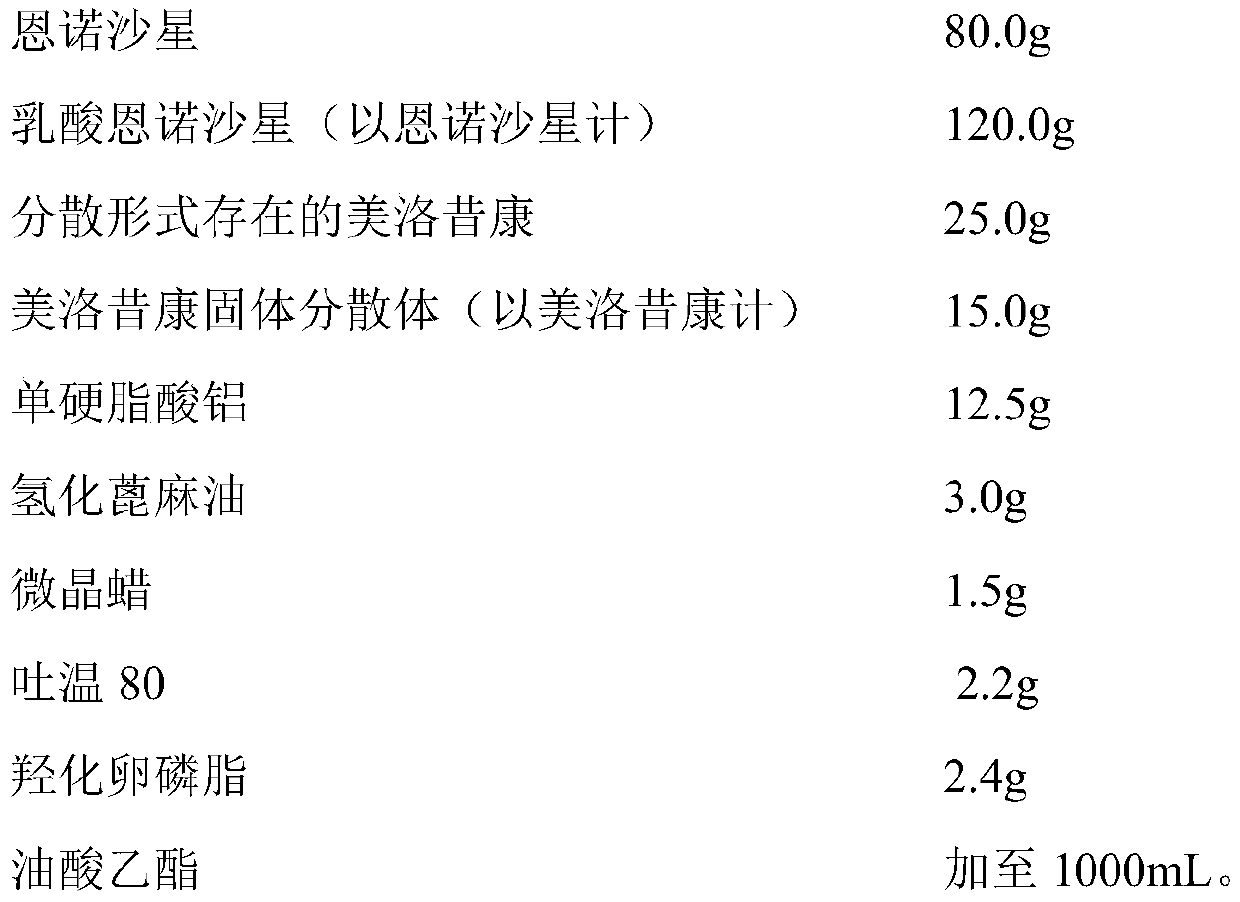
InactiveCN110327348ASimple preparation processGood batch-to-batch repeatabilityAntibacterial agentsOrganic active ingredientsMeloxicamDisease outcome
The present invention discloses a compound long-acting injection containing enrofloxacin and meloxicam. The compound long-acting injection comprises the enrofloxacin and / or a salt thereof, the meloxicam in a dispersion form, a meloxicam solid dispersoid, a high molecular retardant, a wetting agent and a dispersion medium; each 1,000 mL of the injection contains 20-250 g of the enrofloxacin and / orthe salt thereof (calculated by the enrofloxacin), 5-50 g of the meloxicam in the dispersion form, 5-30 g of the high molecular retardant, 5-20 g of the wetting agent, and the balance of a dispersionmedium; and a mass ratio of the meloxicam in the dispersion form to the meloxicam solid dispersoid (calculated by the meloxicam) is (5:1)-(1:1). The injection preparation is stable in quality, slow indrug release, safe and effective, only 1-2 times of drug administration in one course of treatment can treat both symptoms and root causes, and effective blood drug concentration is maintained for 3-7 days; and only 1 needle is used for injection for 1 time, so that the compound long-acting injection enhances animal compliance, reduces animal stress and is more favorable for disease outcome and prognosis.
Owner:余祖功
Marker of Prostate Cancer
InactiveUS20140249041A1Increasing transcriptionalImprove translationOrganic active ingredientsPeptide/protein ingredientsDisease outcomeProstate cancer
An SLC18A2 gene serves as a marker of prostate cancer. Methods are provided for diagnosing prostate cancer, predicting or prognosticating the disease outcome, predicting recurrence following surgery, and monitoring disease progression in an individual having prostate cancer. The methods relate to determining the methylation state of an SLC18A2 gene and / or determining the level of transcription or translation of the gene in a sample from the individual. Methods of treating prostate cancer are also provided. The invention also pertains to compositions and kits for use in the methods.
Owner:REGION MIDTJYLLAND +1
Detection of chromosomal abnormalities associated with prognosis of non small cell lung cancer
InactiveUS20120264635A1Increased riskPoor disease outcomeNucleotide librariesMicrobiological testing/measurementDisease outcomeIncreased risk
The methods and compositions described herein address the need for a diagnostic method that can be provided to patients with early stage lung cancer, especially non-small cell lung cancer (NSCLC), to determine whether the patient is at increased risk of poor disease outcome. The methods and compositions thus allow for more informed treatment decisions for the early stage lung cancer patient.
Owner:ABBOTT MOLECULAR INC
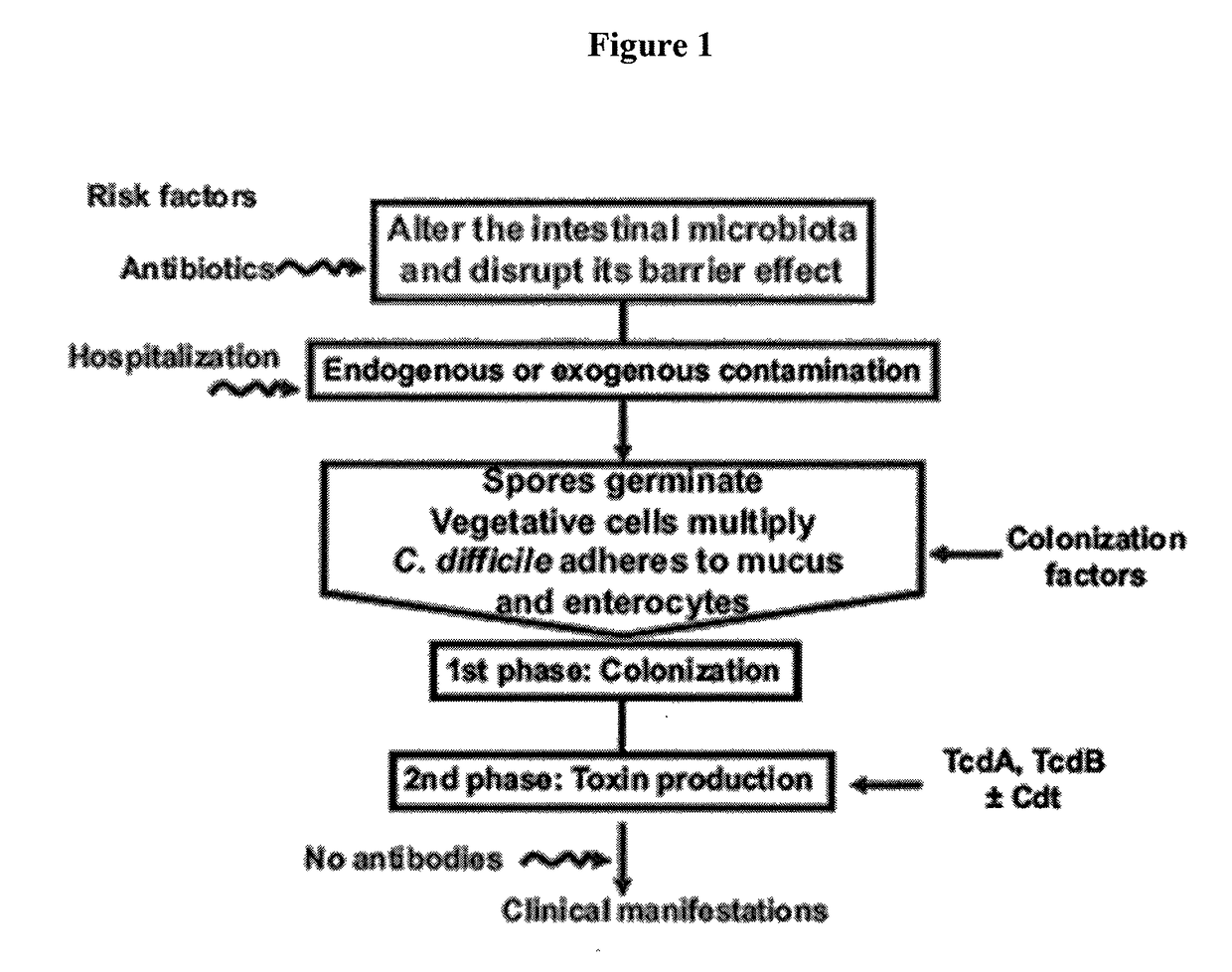
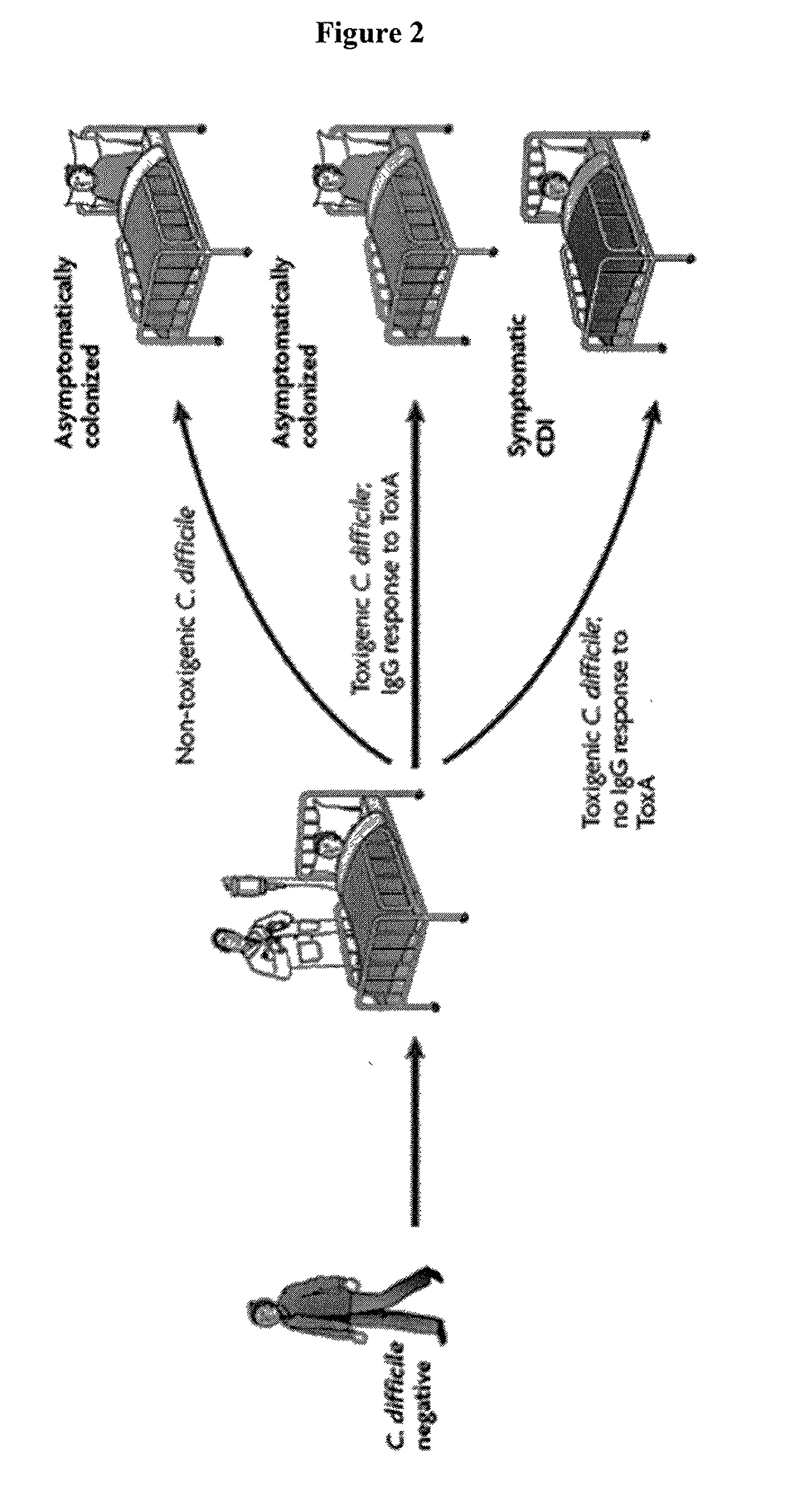
Predictive value of clostridium difficile-specific immune response for recurrence and disease outcome
The present invention relates to the field of Clostridium difficile infections. Methods of diagnosing, treating and preventing Clostridium difficile-associated disease and recurrent CDAD are disclosed herein.
Owner:UNIV PARIS SACLAY +3
Modeling method of mouse hypertensive cerebral hemorrhage model
PendingCN113648100ASurvival rate does not decreaseMeet the experimental needsSurgical veterinaryLeft ventricle wallSkull surface
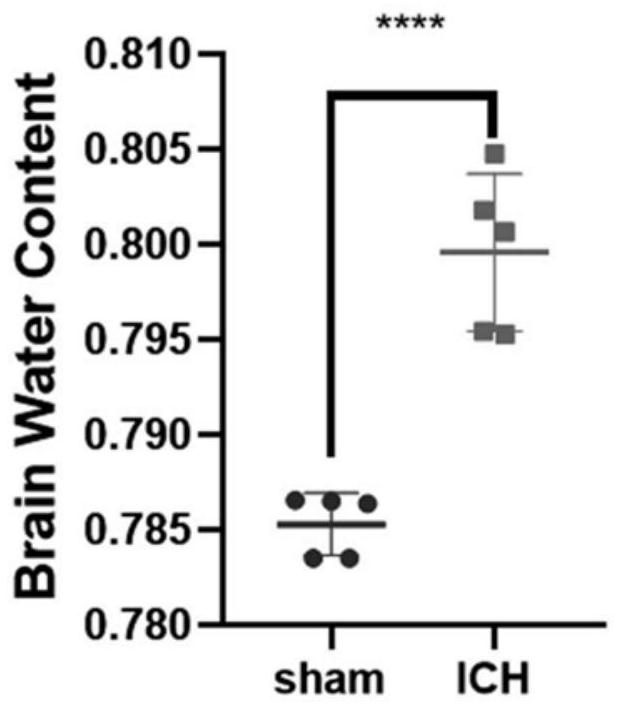
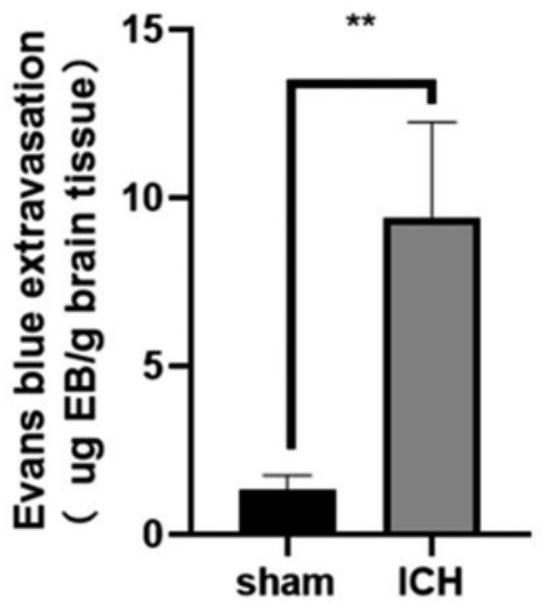
The invention discloses a modeling method of a mouse hypertensive cerebral hemorrhage model, which comprises the following steps of: puncturing and taking blood from the left ventricle of a mouse, exposing a skull surgery field by wiping and cleaning soft tissues covering the skull, drilling after determining a needle insertion point on the surface of the skull, injecting autologous blood at the drilling position through a brain stereotaxic instrument, and then injecting the autologous blood into the mouse hypertensive cerebral hemorrhage model; and after injection is completed, sealing the drilled hole with bone wax, disinfecting the incision, and suturing the skin. According to the method, arterial blood is taken through left ventricle puncture, the survival rate of a mouse is not reduced on the premise that sufficient arterial blood is extracted, autologous arterial blood is accurately injected back into the tail shell core of the basal segment in the brain through a mouse brain locator, and stable hematoma is formed. The method provides guarantee for subsequent experiments, is beneficial to experimental contrast research, is very similar to clinical pathological changes and disease outcome of hypertension patients, and has important significance on disease outcome and treatment schemes after cerebral hemorrhage.
Owner:XUZHOU MEDICAL UNIV
Methods for diagnosis and/or prognosis of gynecological cancer
InactiveUS20150024956A1Sugar derivativesMicrobiological testing/measurementDisease progressionMolecular biomarkers
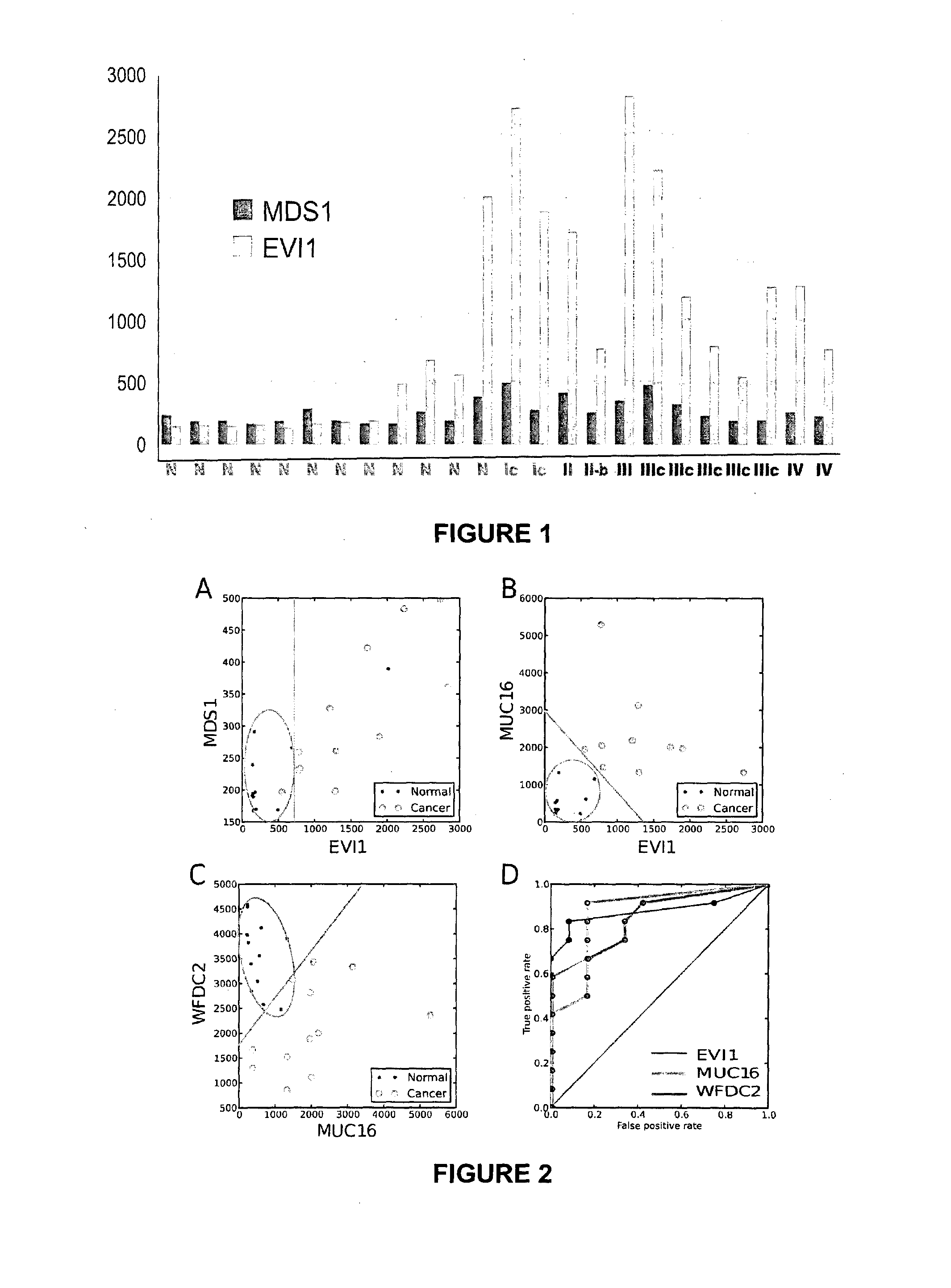
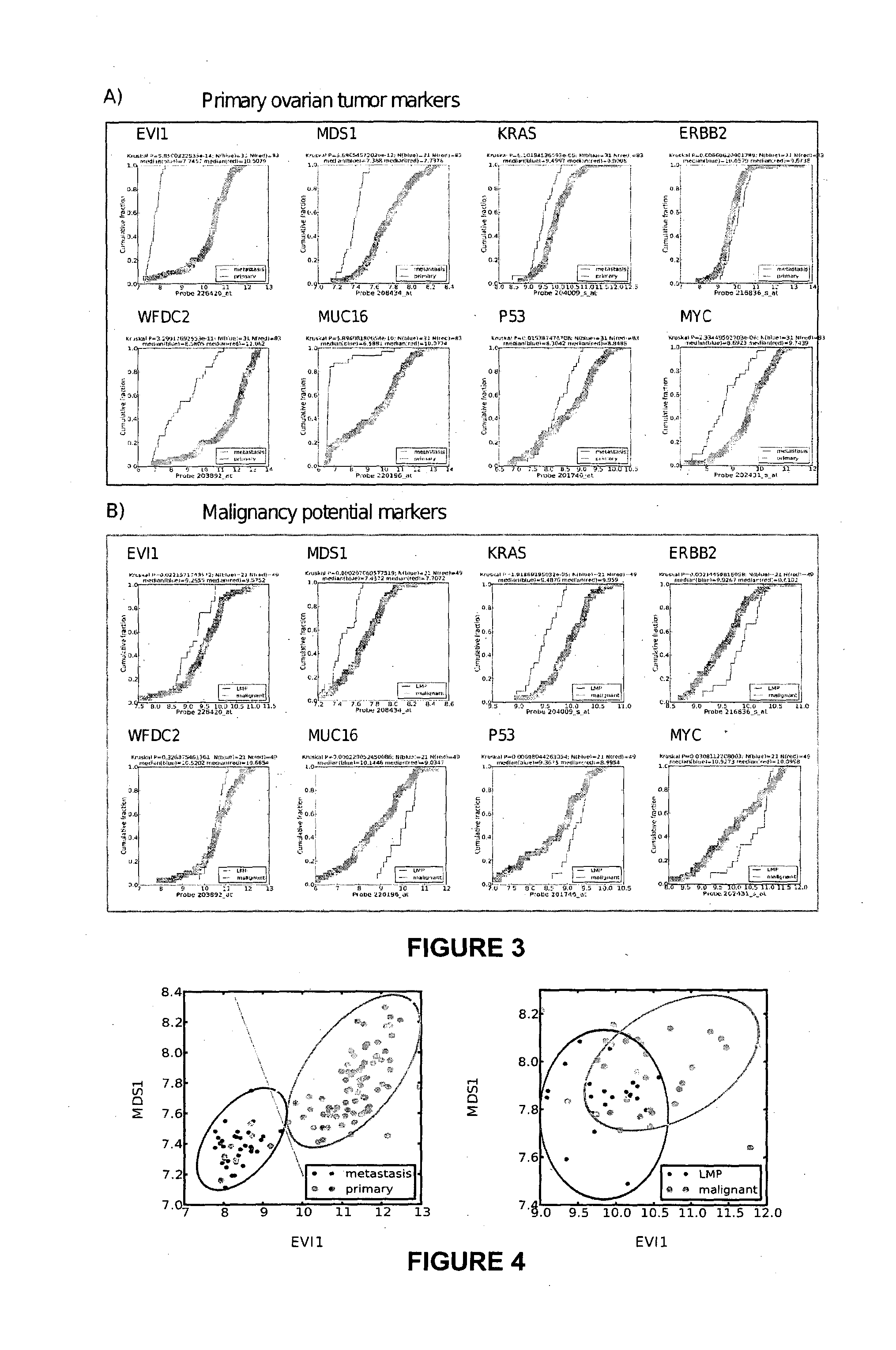
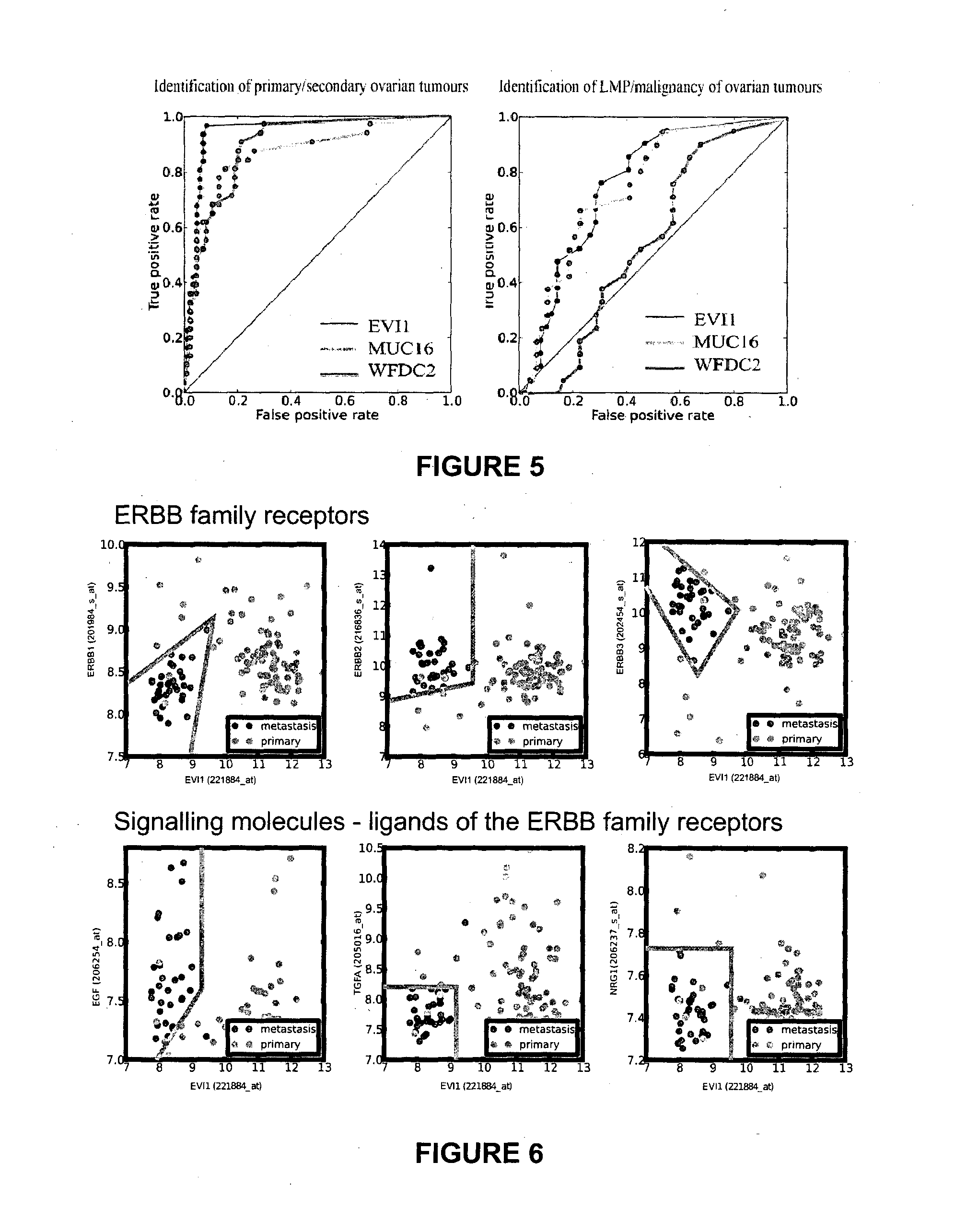
Ovarian, cervical cancer, endometriosis, clear cell renal carcinoma cancers are very heterogeneous diseases which lack robust diagnostic, prognostic and predictive clinical biomarkers. Conventional clinical biomarkers (stages, grades, tumor mass etc) and molecular biomarkers (CA125, KRAS, p53 etc) are not appropriate for early diagnostics, differential diagnostics, prediction and prognosis of the disease outcome for individual patients. The most common type of the human ovarian cancers is human epithelial ovarian cancer (EOC). This cancer is characterized with one of the lowest survival rates compared to other cancers. The present invention relates to an in vitro method for diagnosing epithelial ovarian cancer, cervical cancer, endometriosis, dear cell renal carcinoma and / or predisposition to epithelial ovarian cancer in a subject, the method comprising determining in a sample of the subject gene expression level of at least one gene in the MDS1 and EVI1 complex (MECOM) locus; and / or copy number of at least one gene in the MECOM locus; wherein the level against at least one expression cutoff value and / or copy number against at least one copy number cutoff value are indicative of the subject having epithelial ovarian cancer, cervical cancer, endometriosis, clear cell renal carcinoma and / or a predisposition to epithelial ovarian cancer, cervical cancer, endometriosis, dear cell renal carcinoma and / or determining whether the ovarian cancer in the subject is primary or secondary ovarian cancer and / or a risk of the disease progression after surgery treatment, and / or an effectiveness of post-surgery chemotherapy.
Owner:AGENCY FOR SCI TECH & RES
Methods used in identifying glioblastoma
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE
Use of monoclonal antibodies to distinguish protein conformational isoforms
InactiveUS7932043B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsScreening techniquesPassive Immunizations
Owner:HEINRICH HEINE UNIV OF DUSSELDORF +1
Novel isoform of myotonic dystrophy associated protein kinase and uses thereof
InactiveUS20020061571A1Rapid diagnosis of an ongoing myocardial infarctionQuick upgradeSugar derivativesMicrobiological testing/measurementDisease outcomeProtein kinase domain
Owner:WISCONSIN ALUMNI RES FOUND
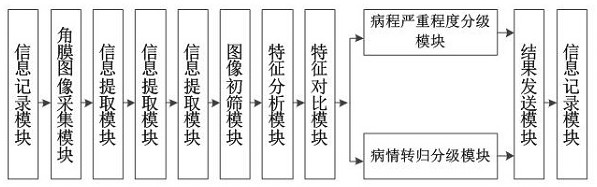
A system for intelligent analysis of corneal nerve fibers using in vivo confocal microscopy images
ActiveCN110310282BEasy accessEasy to recordImage enhancementImage analysisDisease outcomeNerve network
The invention discloses a system for intelligently analyzing corneal nerve fibers by using confocal microscope images in vivo, including: an information recording module; a corneal image acquisition module for collecting several confocal microscope images of each layer of the cornea; an information extraction module; Sieve module, locate confocal images of abnormal corneal tissue from each corneal confocal image through the first neural network model; feature analysis module; feature comparison module; disease grading module, including disease course severity grading module and disease outcome grading module The course severity grading module is that all corneal confocal pictures pass through the second neural network model to obtain the course severity level; Return to grade; result sending module. The invention analyzes the characteristics of corneal nerve fibers, the degree of damage and the changing process thereof, and evaluates the state of local or systemic neuropathy.
Owner:THE PEOPLES HOSPITAL OF GUANGXI ZHUANG AUTONOMOUS REGION
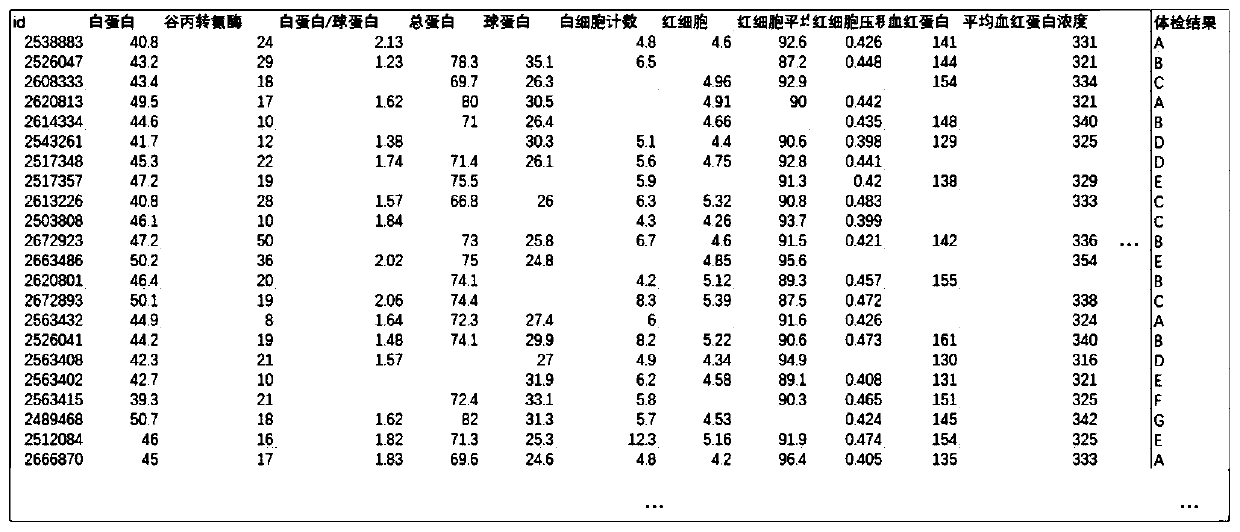
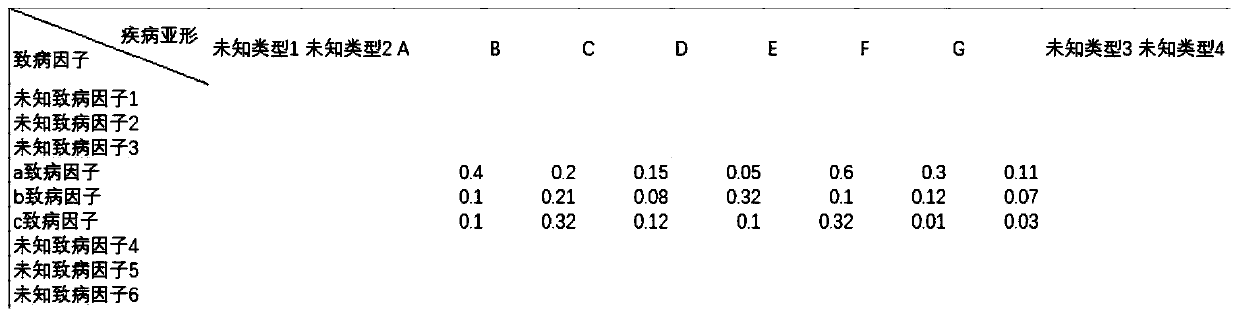
A method, device and application of medical examination data completion based on side information
The invention discloses a physical examination data supplementing method based on edge information. The method comprises the steps of (1), constructing and supplementing a physical examination-diseasematrix, a pathogenic factor-disease matrix, a pathogenic factor-physical examination matrix according to the edge information; (2), establishing coding-decoding networks D2F Net, D2C Net and F2C Netbetween two random matrixes; (3), performed combined training on the D2F Net, D2C Net and F2C Net, wherein after training is finished, the pathogenic factor-physical examination matrix is supplemented; and (4), inputting the to-be-supplemented physical examination-disease matrix into the D2F Net, D2C Net, and by means of the supplemented pathogenic factor-disease matrix, the pathogenic factor-physical examination matrix and the F2C Net, supplementing the physical examination-disease matrix through calculation. The invention further discloses a physical examination data supplementing device based on the edge information, wherein the physical examination data and the disease result can be supplemented according to existing information.
Owner:SHANDONG IND TECH RES INST OF ZHEJIANG UNIV
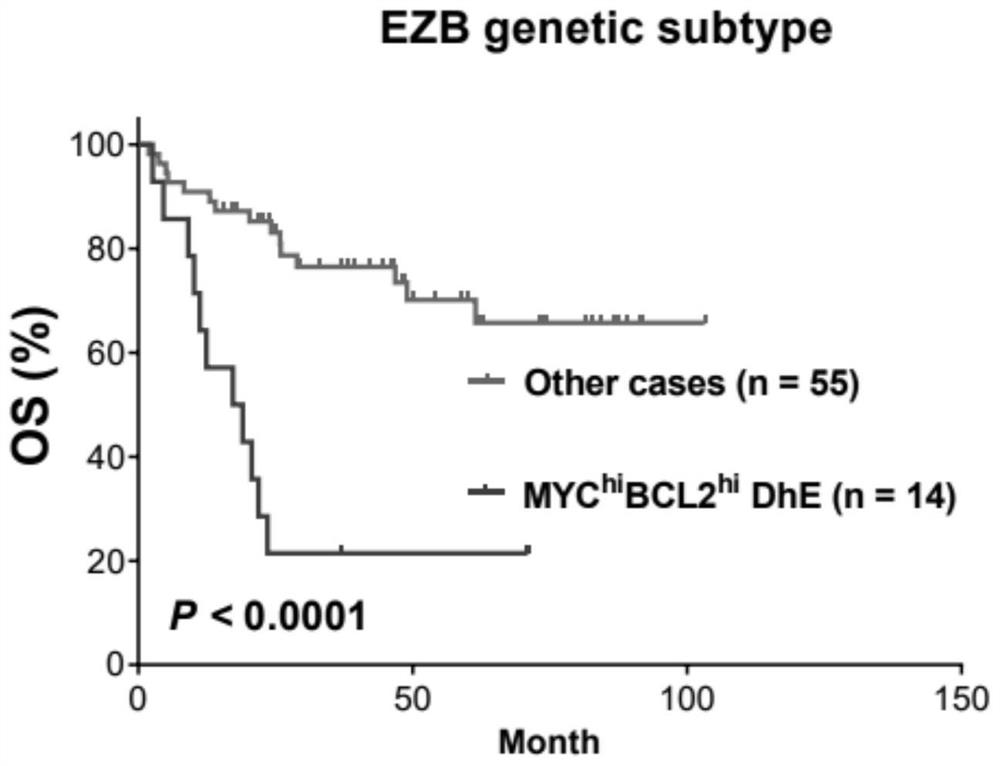
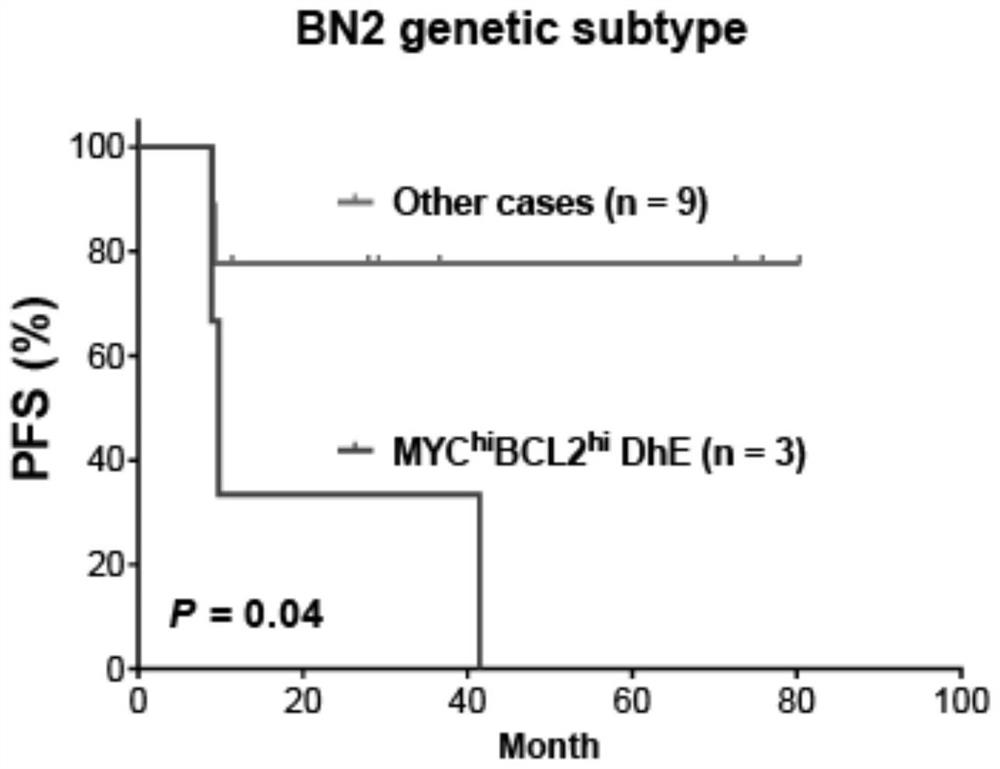
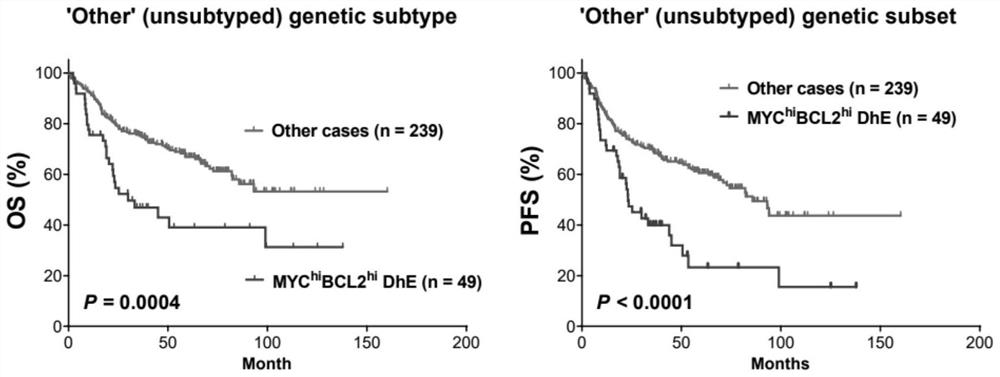
Diffuse large B-cell lymphoma prognosis model based on MYC/BCL2 double expression and immune microenvironment, application and prognosis method
PendingCN114085910APrecise Stratified TreatmentMicrobiological testing/measurementDisease diagnosisDisease outcomeDiffuse large cell lymphoma
The invention discloses a diffuse large B-cell lymphoma prognosis model based on MYC / BCL2 double expression and an immune microenvironment, application and a prognosis method. Based on the MYC / BCL2 protein expression level and the immune microenvironment, the MYC / BCL2 protein expression level and the immune microenvironment synergistically play a unique role in prognosis of various DLBCL subtype patients, assist clinicians in judging clinical development and disease outcome of the patients, and realize precise layered treatment.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com