Modeling method of mouse hypertensive cerebral hemorrhage model
A hypertensive cerebral hemorrhage and mouse technology, applied in the field of biomedicine, can solve problems such as the inability to simulate the pathological changes and disease outcomes of cerebral hemorrhage, the inability to form a hematoma, and the difficulty of puncturing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for building a mouse hypertensive cerebral hemorrhage model, the specific steps are as follows:
[0041] (1) Blood collection by left ventricular puncture: the mice were anesthetized by isoflurane gas induction, the mice were placed in the supine position on the cardboard, the limbs were fixed with medical adhesive tape, the cotton thread was buckled over the front teeth of the mice, and pulled Fix the mouse so that the chest and upper abdomen of the mouse are fully exposed. Use a cotton swab dipped in 75% standard medical alcohol to disinfect the chest and upper abdomen on a large scale, touch the strongest heartbeat (apex) of the chest with the left hand, and then use a 1mL syringe at the angle between the xiphoid process of the sternum and the ribs at an angle of 10° Puncture at the point with the strongest heartbeat at ~20°, draw blood while withdrawing the needle, and quickly transfer the arterial blood to a 1.5mL EP tube for later use.
[0042] (2) Expos...
Embodiment 2
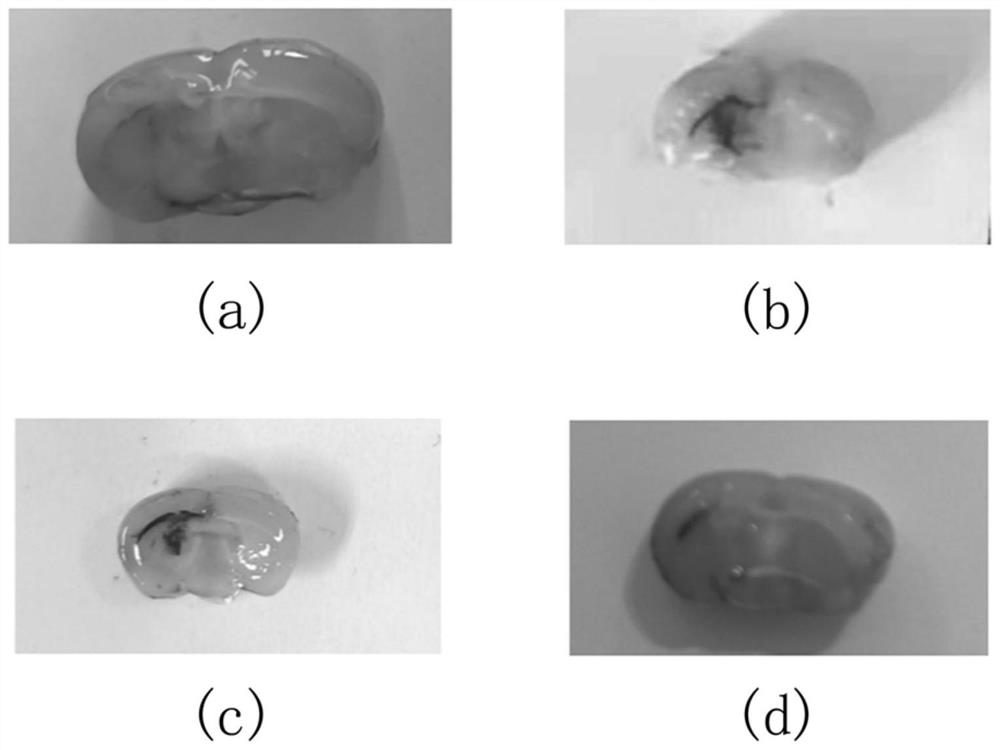
[0048] Example 2 Coronal view of hematoma after hypertensive cerebral hemorrhage modeling in mice
[0049] Ten 5- to 7-week-old male Kunming mice (derived from swiss mice), weighing 20 to 30 g, were divided into two groups (n=5), respectively Sham and ICH groups, raised in a quiet environment and given free access to water Feed. The sham group did not draw autologous blood, but only inserted the microsampler 3.7 mm from the drill hole to the ventral side, kept the needle in place for 10 minutes before pulling it out, then pulled it back at a speed of 1 mm / min, and sealed it with bone wax Holes were drilled and the skin was sutured. The ICH group was performed according to the specific steps of the modeling method described in Example 1. After modeling, the coronal plane of Sham group and the coronal plane of hematoma in ICH group were observed. Coronal incision was made on the coronal plane of ICH group with the injection point as the center point, and 1, 2, and 3 days were ...
Embodiment 3
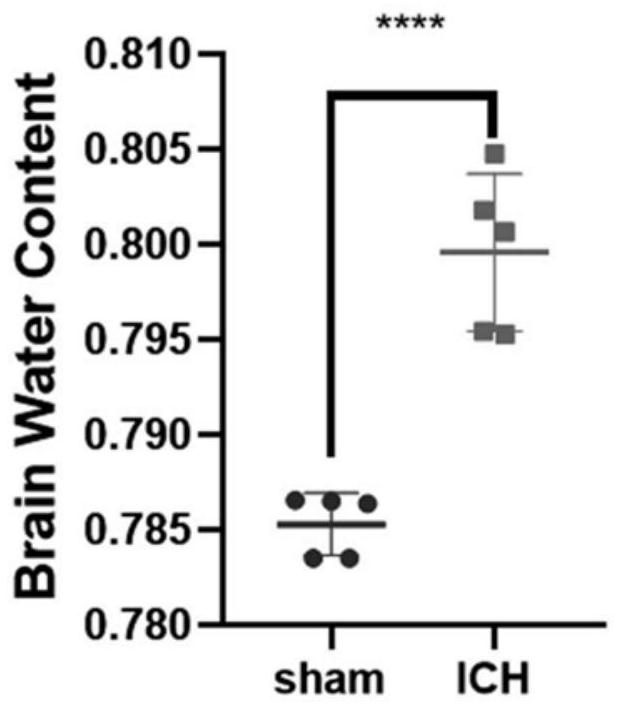
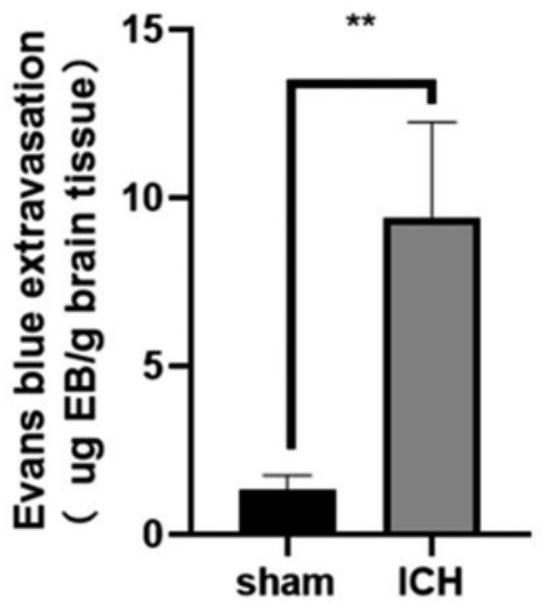
[0051] Example 3 Determination of degree of brain edema after hypertensive intracerebral hemorrhage modeling in mice
[0052] Ten 5- to 7-week-old male Kunming mice, weighing 20-30 g, were divided into two groups (n=5), namely the Sham group and the ICH group, and were reared in a quiet environment with free access to water and food. The sham group did not draw autologous blood, but only inserted the microsampler 3.7 mm from the drill hole to the ventral side, kept the needle in place for 10 minutes before pulling it out, then pulled it back at a speed of 1 mm / min, and sealed it with bone wax Holes were drilled and the skin was sutured. The ICH group was performed according to the specific steps of the modeling method described in Example 1. Then, using the dry-wet weight method, the mice in each group were injected intraperitoneally with 10% chloral hydrate for 24 hours to induce anesthesia, the brain was decapitated, the olfactory bulb, cerebellum and brainstem were removed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com