Compound long-acting injection containing enrofloxacin and meloxicam, and preparation method of compound long-acting injection
A technology of meloxicam and injection, which is applied in the field of compound long-acting injection and its preparation, can solve the problems of inconvenient clinical application, unfavorable drug efficacy, and excessive drinking water waste, so as to enhance animal compliance and reduce animal stress. Exciting and cost-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0034] Enrofloxacin or its salts shall be ultrafinely pulverized, and the particle size D90 shall be ≤5 μm, and no particles above 25 μm shall be detected.
[0035] Meloxicam is ultrafinely pulverized to control the particle size D90≤5μm, and no particles above 25μm can be detected.
[0036] The ultrafine pulverization process is as follows:
[0037] The fluidized bed supersonic airflow superfine pulverization and classification system is adopted, and the material input volume is controlled at 60-80g. The materials collide with each other at the speed of Mach 2.5 in the crushing chamber, and are crushed quickly. The crushed particles rise to the classification chamber with the airflow, and the qualified particles enter the cyclone separator with the airflow, and finally obtain the desired product, and the tail gas enters the dust collector to be discharged. Under the action of the classifier, the larger particles fall back to the crushing chamber and are crushed again until ...
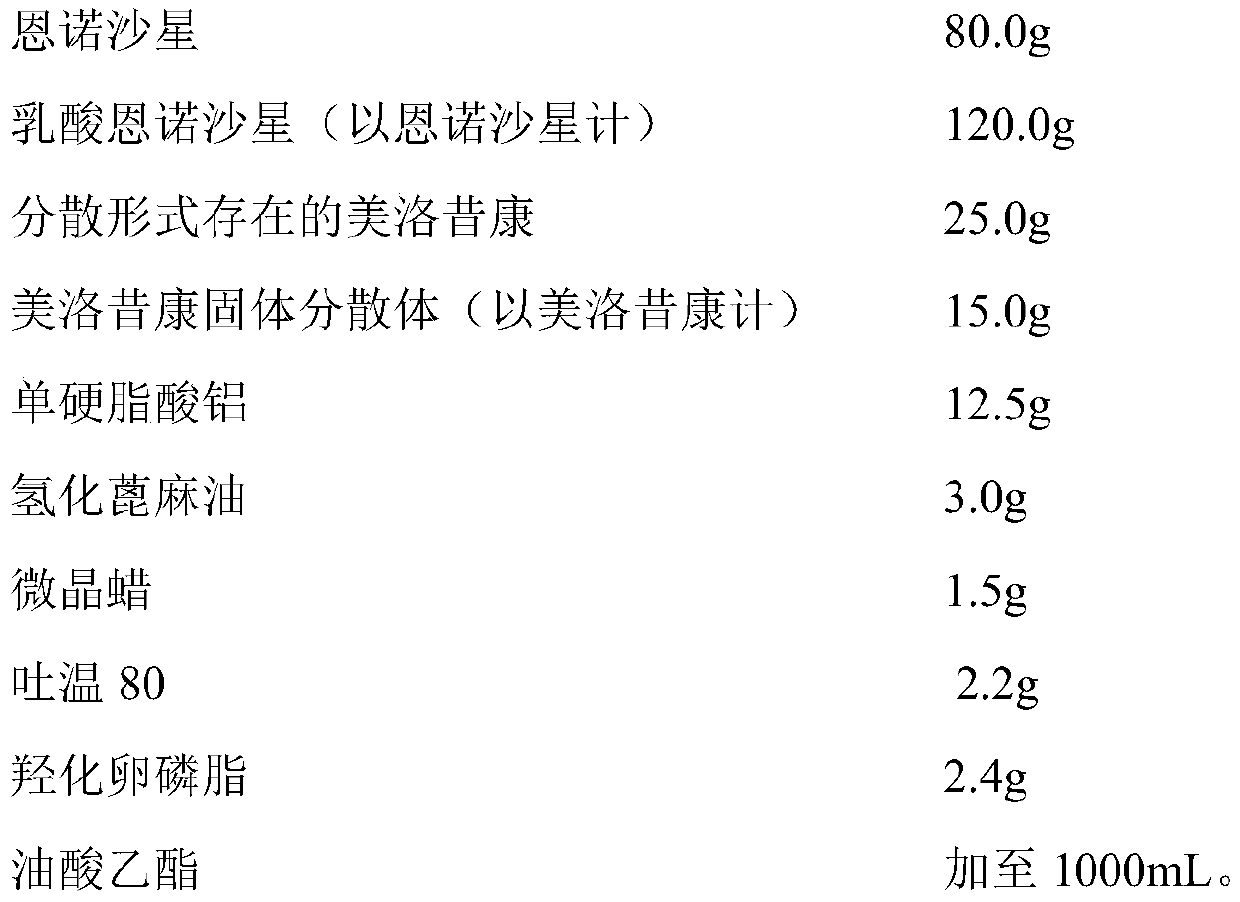
Embodiment 1
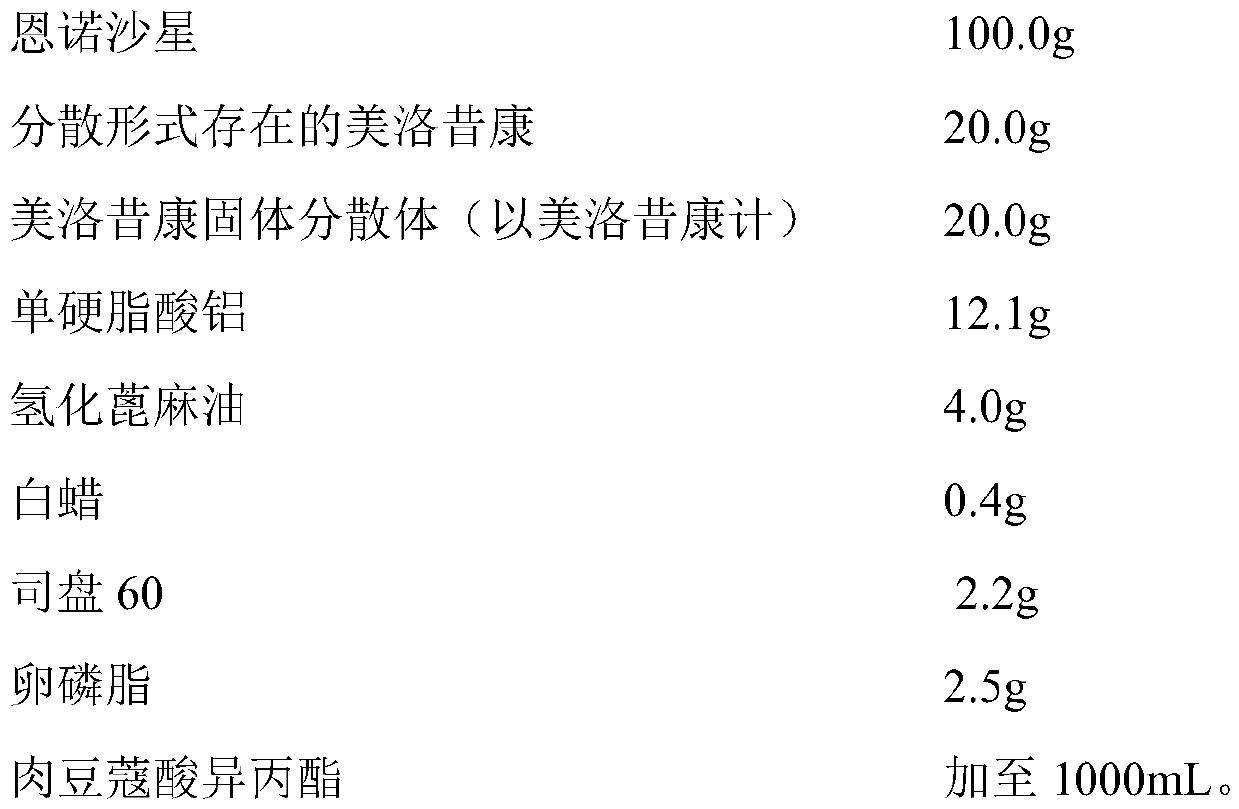
[0039] prescription
[0040]
[0041] Among them, the meloxicam solid dispersion is prepared by the following method: add 100.0g meloxicam to 100.0g dimethylformamide, mix well, and sonicate for 1h; In the molten mixture of 50.0g Poloxamer 188 and 250.0g polyethylene glycol 6000, stir for 0.5h; pour the molten material onto a frozen stainless steel plate, after cooling and solidifying, pulverize and sieve to obtain meloxicam solid Dispersion, spare.
[0042] The preparation method of the long-acting injection containing enrofloxacin and meloxicam:
[0043] Take an appropriate amount of isopropyl myristate, heat it to 150-180°C, and continue for 1 hour; take 700mL of heat-treated isopropyl myristate, cool it down to 120-140°C, add aluminum monostearate, hydrogenated castor oil , maintain 1-2h, make aluminum monostearate, hydrogenated castor oil gel completely; let cool to 40-60 ℃, add white wax, Span 60, lecithin, enrofloxacin, meloxicam solid dispersion and meloxicam in ...
Embodiment 2
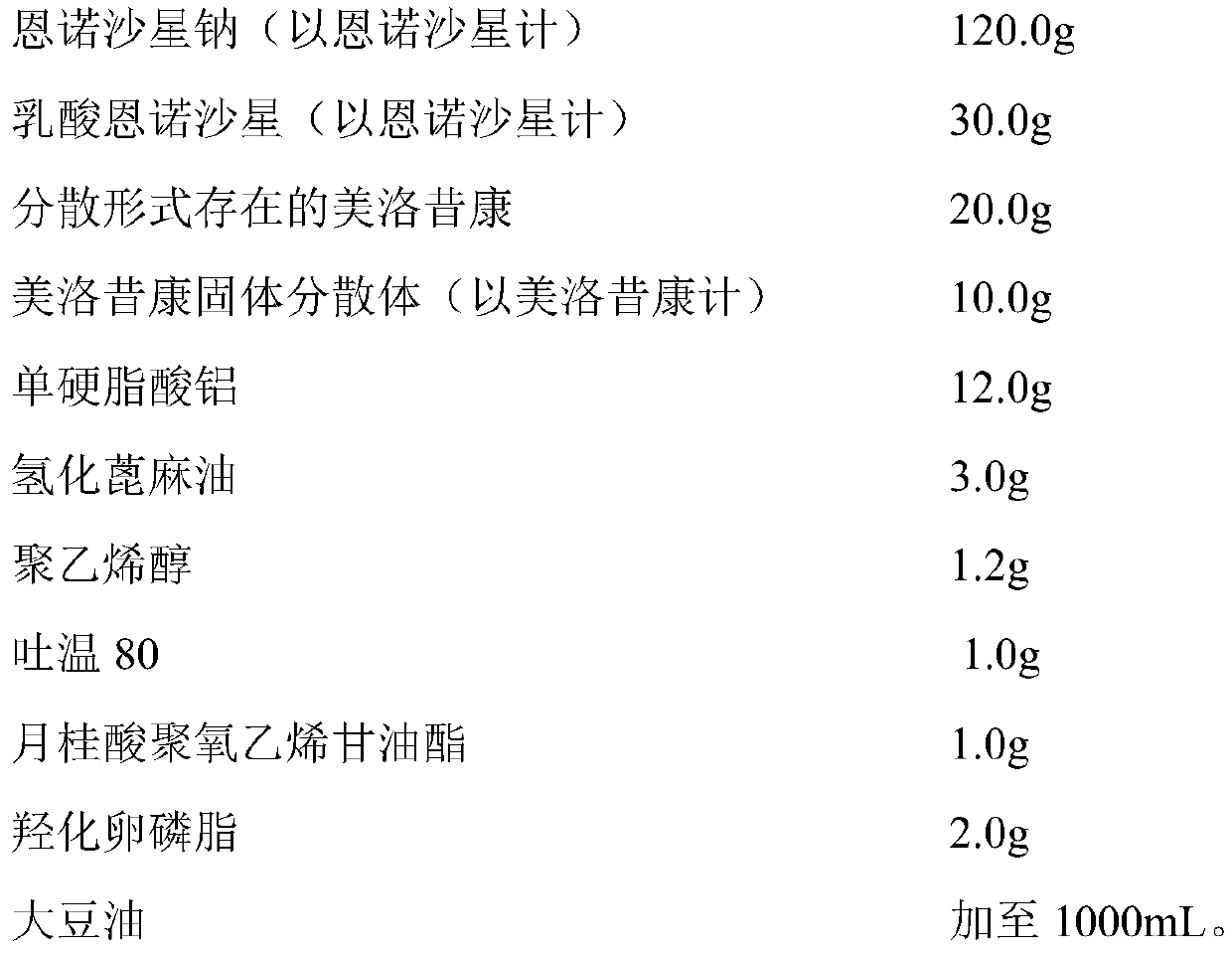
[0050] prescription
[0051]
[0052] Among them, the meloxicam solid dispersion is prepared by the following method: add 100.0g meloxicam to 100.0g dimethylformamide, mix well, and sonicate for 1h; Melt 100.0g of poloxamer 188 and 200.0g of polyethylene glycol 12000 mixture, stir for 1h; pour the molten material onto a frozen stainless steel plate, cool and solidify, pulverize and sieve to obtain meloxicam solid dispersion Body, spare.
[0053] The preparation method of the long-acting injection containing enrofloxacin and meloxicam: take an appropriate amount of soybean oil, heat it to 150-180°C for 1 hour; take 700mL of heat-treated soybean oil, cool it to 120-140°C, Add aluminum monostearate and hydrogenated castor oil and keep for 1-2 hours to completely gel the aluminum monostearate and hydrogenated castor oil; let it cool to 40-60°C, add polyvinyl alcohol, Tween 80, polylauric acid Oxyethylene glyceride, hydroxylated lecithin, enrofloxacin sodium, enrofloxacin lact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com