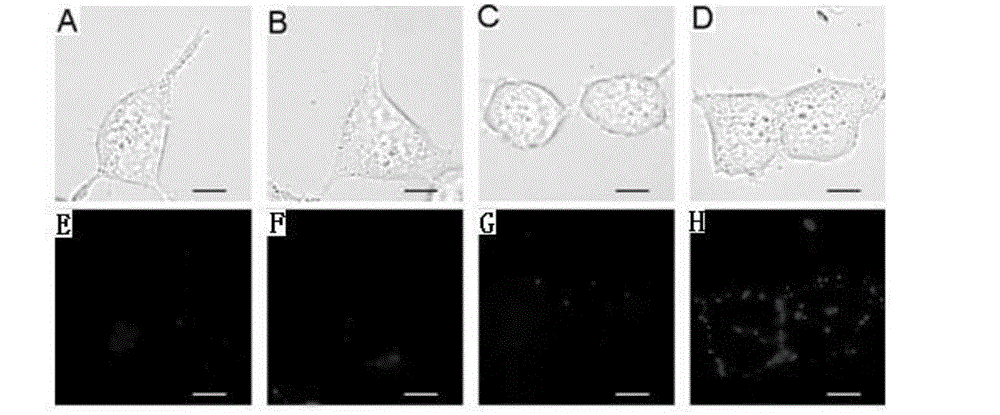
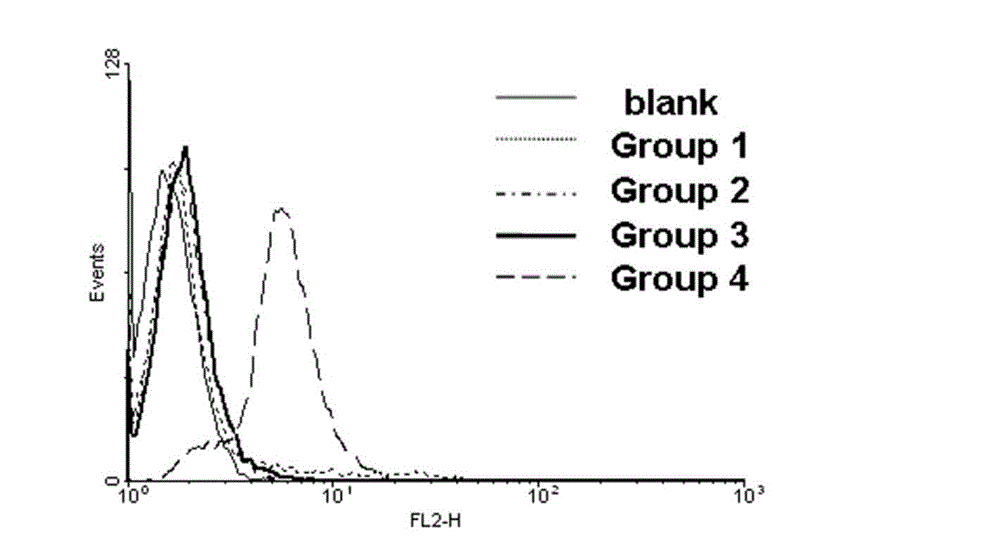
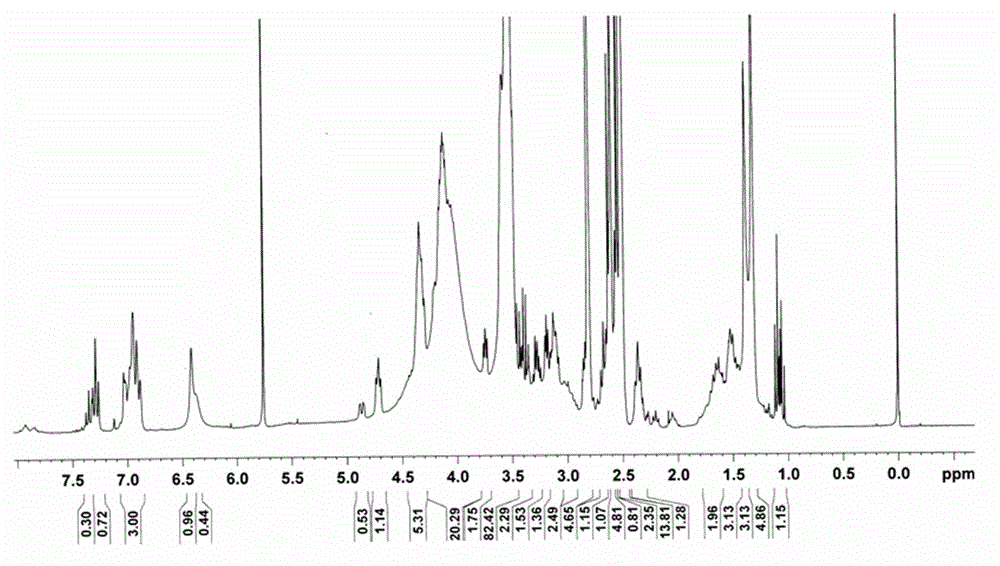
Biotin derivative of phenylephrine and preparation method and application thereof
A technology of phenylephrine and biotin derivatives, which is applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of inability to visualize the dynamic process of cells, lack of such problems, and achieves reduction of intermediate steps, reduction of steric hindrance, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A preparation method of a biotin derivative of phenylephrine, the reaction scheme is as follows:
[0038]
Embodiment 1
[0040] Diisomeric Polyethylene Glycol H 2 Synthesis of N-PEG-COOH
[0041] Add 2.2mL of potassium hexamethyldisilazide and 4.5mL of ethylene oxide into a dry and sealed one-necked bottle, dissolve in 18mL of anhydrous tetrahydrofuran, stir, and react in ice bath for 24 hours, then add 0.50g of succinic anhydride , continue to react for 5-6 hours, finally add excess glacial acetic acid, avoid light and stir for 3-4 hours, drop the reaction solution into a large amount of ether to settle three times, and dry the obtained solid in vacuum at 40°C for 24 hours to obtain a brown solid 3.64 g, yield 95.6%.
Embodiment 2
[0043] Synthesis of t-Boc-phenylephrine
[0044] Protect the amino group of phenylephrine: add 1.21g (6mmol) of phenylephrine hydrochloride to a 25mL single-necked bottle, dissolve with 7mL of 2M NaOH solution, stir with a magnet for 20 minutes and put it in an ice bath. Dissolve 1.47g (6.7mmol) of di-tert-butyl dicarbonate in 1mL of tetrahydrofuran to form a solution, and slowly drop the solution into a one-necked flask with a constant pressure dropping funnel, and accelerate the stirring. Remove the ice bath after the dropwise addition, stir at room temperature, follow the reaction by TLC (thin-layer chromatography analysis), and complete the reaction in 24 hours. Adjust the reaction solution to pH=7 with glacial acetic acid, extract with 15mL×3 dichloromethane, and evaporate to dryness In the dichloromethane layer, 1.39 g of a yellow viscous product was obtained, yield: 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com